We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1652
From Proteopedia
(Difference between revisions)
| Line 64: | Line 64: | ||
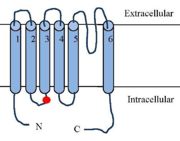
'''Phosphorylation''' of the TRPV1 receptor leads to its sensitization | '''Phosphorylation''' of the TRPV1 receptor leads to its sensitization | ||
| - | (the process regulates TRPV1 its functionality). Phosphorylations are either caused by '''PKC''' ([https://en.wikipedia.org/wiki/Inositol_trisphosphate IP3 signalling]), by '''PKA''' ([https://fr.wikipedia.org/wiki/Adénylate_cyclase AMPc signalling]), or by '''CamKII'' activation by inflammatory mediators. <ref name="Integrating TRPV1 Receptor Function with Capsaicin Psychophysics"> <ref>K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.</ref> Depending on the target residu, the impact on the receptor will be different as various activation pathways are impacted. Phosphorylation improves the efficiency of the receptor and because it regulates the function, it is a target to treat [https://en.wikipedia.org/wiki/Hyperalgesia]. <ref name="Phosphorylation of TRPV1 S801 Contributes to Modality-Specific Hyperalgesia in Mice"> <ref>John Joseph, L. Qu, S. Wang, M. Kim, D. Bennett, J. Ro, M. J. Caterina and MK. Chung, Journal of Neuroscience 11 December 2019, 39 (50) 9954-9966. https://www.jneurosci.org/content/39/50/9954 (Consulté le: déc. 23, 2021). [En ligne].</ref>. PKA phosphorylates <scene name='86/868185/S502_t370/1'>T370 and S502</scene>, PKC and CaMKII phosphorylate <scene name='86/868185/Ser502_thr704/1'>S502 and T704</scene>. | + | (the process regulates TRPV1 its functionality). Phosphorylations are either caused by '''PKC''' ([https://en.wikipedia.org/wiki/Inositol_trisphosphate IP3 signalling]), by '''PKA''' ([https://fr.wikipedia.org/wiki/Adénylate_cyclase AMPc signalling]), or by '''CamKII''' activation by inflammatory mediators. <ref name="Integrating TRPV1 Receptor Function with Capsaicin Psychophysics"> <ref>K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.</ref> Depending on the target residu, the impact on the receptor will be different as various activation pathways are impacted. Phosphorylation improves the efficiency of the receptor and because it regulates the function, it is a target to treat [https://en.wikipedia.org/wiki/Hyperalgesia Hyperalgesia]. <ref name="Phosphorylation of TRPV1 S801 Contributes to Modality-Specific Hyperalgesia in Mice"> <ref>John Joseph, L. Qu, S. Wang, M. Kim, D. Bennett, J. Ro, M. J. Caterina and MK. Chung, Journal of Neuroscience 11 December 2019, 39 (50) 9954-9966. https://www.jneurosci.org/content/39/50/9954 (Consulté le: déc. 23, 2021). [En ligne].</ref>. PKA phosphorylates <scene name='86/868185/S502_t370/1'>T370 and S502</scene>, PKC and CaMKII phosphorylate <scene name='86/868185/Ser502_thr704/1'>S502 and T704</scene>. |
The phosphorylation of TRPV1 lead to an '''over-expression ''' of TRPV1 at the membrane surface.<ref>K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.</ref> Moreover, phosphorylated TRPV1 would have a reduced channel opening threshold.<ref>G. Bhave et al., « Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) », Proc. Natl. Acad. Sci., vol. 100, no 21, p. 12480‑12485, oct. 2003, doi: 10.1073/pnas.2032100100.</ref>. As a result phosphorylated TRPV1 are more responsive to agonist and the resulting pain sensation is higher. | The phosphorylation of TRPV1 lead to an '''over-expression ''' of TRPV1 at the membrane surface.<ref>K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.</ref> Moreover, phosphorylated TRPV1 would have a reduced channel opening threshold.<ref>G. Bhave et al., « Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) », Proc. Natl. Acad. Sci., vol. 100, no 21, p. 12480‑12485, oct. 2003, doi: 10.1073/pnas.2032100100.</ref>. As a result phosphorylated TRPV1 are more responsive to agonist and the resulting pain sensation is higher. | ||
Revision as of 09:52, 30 December 2021
| This Sandbox is Reserved from 26/11/2020, through 26/11/2021 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1643 through Sandbox Reserved 1664. |
To get started:
More help: Help:Editing |
The Transient Receptor Potential cation channel subfamily V member 1 TRPV1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Wikipedia contributors. (2020, december 21). TRPV1. Wikipedia. https://en.wikipedia.org/wiki/TRPV1 (Consulted the: dec. 28, 2020). [Online].
- ↑ 2.0 2.1 A. Danigo, L. Magy et C. Demiot , Med Sci (Paris) Volume 29, Number 6-7, Juin–Juillet 2013, p. 597-606. TRPV1 dans les neuropathies douloureuses, https://www.medecinesciences.org/en/articles/medsci/full_html/2013/08/medsci2013296-7p597/medsci2013296-7p597.html, (Consulted the : dec. 23, 2021). [Online].
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Liao, M., Cao, E., Julius, D., & Cheng, Y. (2013b). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature, 504(7478), 107‑112. https://doi.org/10.1038/nature12822(consulté le déc. 28, 2020)
- ↑ T. Rosenbaum et S. A. Simon, « TRPV1 Receptors and Signal Transduction », in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, W. B. Liedtke et S. Heller, Éd. Boca Raton (FL): CRC Press/Taylor & Francis, 2007
- ↑ 5.0 5.1 5.2 5.3 5.4 G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016
- ↑ R. Kumar, A. Hazan, A. Basu, N. Zalcman, H. Matzner, et A. Priel, « Tyrosine Residue in the TRPV1 Vanilloid Binding Pocket Regulates Deactivation Kinetics », J. Biol. Chem., vol. 291, no 26, p. 13855‑13863, juin 2016, doi: 10.1074/jbc.M116.726372.
- ↑ X. Yao, H.-Y. Kwan, et Y. Huang, « Regulation of TRP Channels by Phosphorylation », Neurosignals, vol. 14, no 6, p. 273‑280, 2005, doi: 10.1159/000093042
- ↑ F. Yang et J. Zheng, « Understand spiciness: mechanism of TRPV1 channel activation by capsaicin », Protein Cell, vol. 8, no 3, p. 169‑177, mars 2017, doi: 10.1007/s13238-016-0353-7.
- ↑ F. Yang et al., « Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel », Nat. Chem. Biol., vol. 11, no 7, Art. no 7, juill. 2015, doi: 10.1038/nchembio.1835.
- ↑ F. Yang et al., « The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel », Nat. Commun., vol. 9, no 1, Art. no 1, juill. 2018, doi: 10.1038/s41467-018-05339-6.
- ↑ 11.0 11.1 K. Elokely et al., « Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin », Proc. Natl. Acad. Sci., vol. 113, no 2, p. E137‑E145, janv. 2016, doi:10.1073/pnas.1517288113.
- ↑ <ref>John Joseph, L. Qu, S. Wang, M. Kim, D. Bennett, J. Ro, M. J. Caterina and MK. Chung, Journal of Neuroscience 11 December 2019, 39 (50) 9954-9966. https://www.jneurosci.org/content/39/50/9954 (Consulté le: déc. 23, 2021). [En ligne].</li> <li id="cite_note-12">[[#cite_ref-12|↑]] K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.</li> <li id="cite_note-13">[[#cite_ref-13|↑]] G. Bhave et al., « Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) », Proc. Natl. Acad. Sci., vol. 100, no 21, p. 12480‑12485, oct. 2003, doi: 10.1073/pnas.2032100100.</li> <li id="cite_note-TRPV1_dans_les_neuropathies_douloureuses_-_Des_mod.C3.A8les_animaux_aux_perspectives_th.C3.A9rapeutiques-14">↑ <sup>[[#cite_ref-TRPV1_dans_les_neuropathies_douloureuses_-_Des_mod.C3.A8les_animaux_aux_perspectives_th.C3.A9rapeutiques_14-0|15.0]]</sup> <sup>[[#cite_ref-TRPV1_dans_les_neuropathies_douloureuses_-_Des_mod.C3.A8les_animaux_aux_perspectives_th.C3.A9rapeutiques_14-1|15.1]]</sup> A. Danigo, L. Magy, et C. Demiot, « TRPV1 dans les neuropathies douloureuses - Des modèles animaux aux perspectives thérapeutiques », médecine/sciences, vol. 29, no 6‑7, Art. no 6‑7, juin 2013, doi: 10.1051/medsci/2013296012.</li></ol></ref>