Dihydrofolate reductase
From Proteopedia
| Line 1: | Line 1: | ||
| - | The enzyme [[dihydrofolate reductase]] (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans<ref>DOI:10.1146/annurev.biophys.33.110502.133613</ref>. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing anti-cancer chemotherapies, while the bacterial enzymes are targets for developing antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics. | + | The enzyme [[dihydrofolate reductase]] (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans<ref>DOI:10.1146/annurev.biophys.33.110502.133613</ref>. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing inhibitors used in anti-cancer chemotherapies, while the bacterial enzymes are targets for developing inhibitors as antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics. |
| + | |||
| + | <ref>DOI:10.1021/acs.biochem.6b01268</ref> | ||
| + | <ref>DOI:</ref> | ||
| + | <ref>DOI:</ref> | ||
| + | <ref>DOI:</ref> | ||
| + | <ref>DOI:</ref> | ||
| + | <ref>DOI:</ref> | ||
| + | <ref>DOI:</ref> | ||
| + | <ref>DOI:</ref> | ||
__TOC__ | __TOC__ | ||
| Line 6: | Line 15: | ||
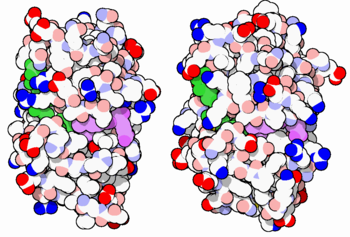
[[Image:34-DihydrofolateReductase-3dfr-1dls.PNG|thumb|350px|E. coli (left) and human (right) DHFR have a similar architecture and mode of binding to NADPH(green) and the competitive inhibitor methotrexate(purple). Original image by David Goodsell]] | [[Image:34-DihydrofolateReductase-3dfr-1dls.PNG|thumb|350px|E. coli (left) and human (right) DHFR have a similar architecture and mode of binding to NADPH(green) and the competitive inhibitor methotrexate(purple). Original image by David Goodsell]] | ||
| - | DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues, and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria. Mice and rats lack the second gene but also show DHFR activity in mitochondria. | + | DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[https://www.proteinatlas.org/ENSG00000228716-DHFR], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria<ref>DOI:10.1073/pnas.1103605108</ref>. Mice and rats lack the second gene but also show DHFR activity in mitochondria<ref>DOI:10.1016/j.febslet.2015.05.017</ref>. |
== Reactions catalyzed == | == Reactions catalyzed == | ||
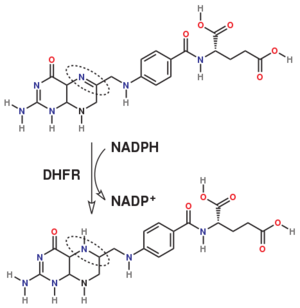
| - | [[Dihydrofolate reductase]] (DHFR) is an enzyme which uses the co-factor NADPH as electron donor which converts it to NADP. It catalyzes the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF). The mammalian enzymes also accept folic acid as a substrate, reducing it to THF. This allows the use of folic acid, which is easier to synthesize than DHF or THF, to fortify food. | + | [[Dihydrofolate reductase]] (DHFR, 1.5.1.3 [https://enzyme.expasy.org/EC/1.5.1.3]) is an enzyme which uses the co-factor NADPH as electron donor which converts it to NADP. It catalyzes the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF). The mammalian enzymes also accept folic acid as a substrate, reducing it to THF. This allows the use of folic acid, which is easier to synthesize than DHF or THF, to fortify food.<ref>DOI:10.3746/pnf.2014.19.4.247</ref>. Some bacterial enzymes also accept folic acid as a substrate <ref>DOI:10.1021/acs.biochem.6b01268</ref> but it acts as a competivite inhibitor in the E. coli enzyme. |
| + | |||
| + | |||
[[Image:467px-DHFR rxn.svg.png|300px]] | [[Image:467px-DHFR rxn.svg.png|300px]] | ||
| Line 21: | Line 32: | ||
==Your Heading Here (maybe something like 'Structure')== | ==Your Heading Here (maybe something like 'Structure')== | ||
| - | <StructureSection load='' size='350' side='right' caption='' scene=''> | + | <StructureSection load='' size='350' side='right' caption='' scene='82/82636/Dhfr_ternary/4'> |
== Structure and Function == | == Structure and Function == | ||
| Line 40: | Line 51: | ||
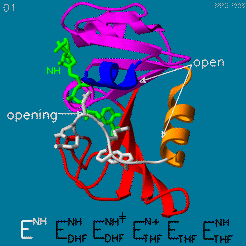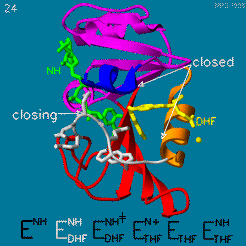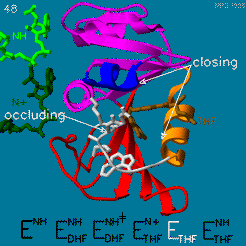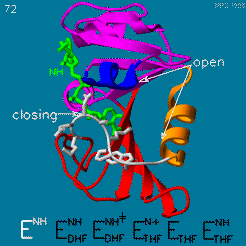
The domain orientation and the Met20 loop conformations changes during the catalytic cycle. A model by M. R. Sawaya and J. Kraut from 1997 summarizing these motions based on six isomorphous crystal structures is shown in the movie below. The original web page of this movie is available on the [https://web.archive.org/web/19991003061611/http://chem-faculty.ucsd.edu/kraut/dhfr.html web archive]. | The domain orientation and the Met20 loop conformations changes during the catalytic cycle. A model by M. R. Sawaya and J. Kraut from 1997 summarizing these motions based on six isomorphous crystal structures is shown in the movie below. The original web page of this movie is available on the [https://web.archive.org/web/19991003061611/http://chem-faculty.ucsd.edu/kraut/dhfr.html web archive]. | ||
| + | |||
| + | The hydride transfer is thought to involve hydride tunneling, supported by temperature-dependent kinetic isotope effects. Tunneling is a quantum phenomenon explaining how a small particle can cross an activation barrier even when it lacks sufficient activation energy. <ref>DOI:10.3390/quantum3010006</ref> | ||
| + | |||
[[Image:Dhfr.movie2.gif]] | [[Image:Dhfr.movie2.gif]] | ||
Revision as of 22:24, 6 January 2022
The enzyme dihydrofolate reductase (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans[1]. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing inhibitors used in anti-cancer chemotherapies, while the bacterial enzymes are targets for developing inhibitors as antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics.
[2] [3] [4] [5] [6] [7] [8] [9]
Contents |
Sources
DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[1], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria[10]. Mice and rats lack the second gene but also show DHFR activity in mitochondria[11].
Reactions catalyzed
Dihydrofolate reductase (DHFR, 1.5.1.3 [2]) is an enzyme which uses the co-factor NADPH as electron donor which converts it to NADP. It catalyzes the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF). The mammalian enzymes also accept folic acid as a substrate, reducing it to THF. This allows the use of folic acid, which is easier to synthesize than DHF or THF, to fortify food.[12]. Some bacterial enzymes also accept folic acid as a substrate [13] but it acts as a competivite inhibitor in the E. coli enzyme.
The folate is a form of the essential vitamin B9.
Relevance
DHFR forms a complex with thymidylate synthase (TS)[14]. Both enzymes participate in the biosynthesis of pyrimidine.[15]
Your Heading Here (maybe something like 'Structure')
| |||||||||||
See also
Relevance
Antifolate inhibitors like Methotrexate (MTX) which is very similar to folic acid are used in cancer therapy.
3D Structures of Dihydrofolate reductase
Dihydrofolate reductase 3D structures
Additional Resources
- For additional information, see: Cancer.
- See also Molecular Playground/DHFR.
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alexander Berchansky, Joel L. Sussman, Tzvia Selzer, Jaime Prilusky, Eric Martz, Eran Hodis, David Canner