We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1653
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<references/><StructureSection load='5z10' size='350' side='right' caption='Structure of the mechanosensitive Piezo1 channel 1 from [http://www.rcsb.org/structure/5Z10 PBD]' scene=''> | <references/><StructureSection load='5z10' size='350' side='right' caption='Structure of the mechanosensitive Piezo1 channel 1 from [http://www.rcsb.org/structure/5Z10 PBD]' scene=''> | ||
Piezo1 proteins constitute a family of excitatory [[ion channels]] directly gated by mechanical forces. Piezo1 is functionally conserved and very important because all living organisms are subjected to mechanical forces from their environment for instance [https://en.wikipedia.org/wiki/Proprioception proprioception], [https://en.wikipedia.org/wiki/Osmoregulation osmoregulation], vascular tone, blood flow regulation, muscle [https://en.wikipedia.org/wiki/Homeostasis homeostasis], flow sensing in kidney, bladder and lungs.<ref name="Ion Permeation"> DOI 10.1016/j.neuron.2016.01.046 </ref>,<ref name = "Cell Press"> DOI 10.1016/j.cub.2017.01.048 </ref> | Piezo1 proteins constitute a family of excitatory [[ion channels]] directly gated by mechanical forces. Piezo1 is functionally conserved and very important because all living organisms are subjected to mechanical forces from their environment for instance [https://en.wikipedia.org/wiki/Proprioception proprioception], [https://en.wikipedia.org/wiki/Osmoregulation osmoregulation], vascular tone, blood flow regulation, muscle [https://en.wikipedia.org/wiki/Homeostasis homeostasis], flow sensing in kidney, bladder and lungs.<ref name="Ion Permeation"> DOI 10.1016/j.neuron.2016.01.046 </ref>,<ref name = "Cell Press"> DOI 10.1016/j.cub.2017.01.048 </ref> | ||
| - | |||
| - | == Function == | ||
| - | ==='''The different forces perceived by Piezo1'''=== | ||
| - | |||
| - | Piezo1 is a [https://en.wikipedia.org/wiki/Mechanosensitive_channels mechanosensitive channel] which means, it can sense external mechanical forces such as fluid flow-induced shear stress, osmotic stress, and pressure-induced membrane stretch. Moreover, “studies have demonstrated wide expression of the Piezo1 channel that enables many different types of cells to sense a diversity of “outside-in” mechanical forces, including indentation, membrane stretch, shear stress, osmotic stress, ultrasound, and compression”.<ref name= "Adenosine"> DOI 10.3389/fphar.2019.01304</ref> Since Piezo1 channel could also be activated by traction forces, there are two different mechanisms that have been proposed to explain the mechanical activation of Piezo1 channel. These mechanisms are called “force-from-lipids” and “force-from-filaments”.<ref name= "Adenosine" /> | ||
| - | |||
| - | For the “force-from-lipids” mechanism, membrane tension is induced by mechanical forces. This membrane tension leads to a reorganization of lipids within and surrounding the channel proteins. This reorganization of lipids results into membrane lipid-channel protein interactions that induce the channel to open. We can note that a recent study (Lin et al., 2019)<ref name= "Lin"> DOI 10.1038/s41586-019-1499-2 </ref> gave support to this mechanism. | ||
| - | |||
| - | The “force-from-filaments” mechanism proposes that conformational changes occur thanks to interactions between the channel and extracellular matrix or intracellular cytoskeletal proteins resulting in the channel opening.<ref name= "Adenosine"> DOI 10.3389/fphar.2019.01304</ref> | ||
| - | |||
| - | ==='''The wide variety of Piezo1’s functions'''=== | ||
| - | |||
| - | Cells are able to perceive the filling of the stomach or the bladder, blood flow and lungs inflation. | ||
| - | |||
| - | Piezo1 is a sensor of mechanical forces in [https://en.wikipedia.org/wiki/Endothelium endothelial], urothelial and renal epithelial cells. For instance, Piezo1 is involved in shear stress sensing in blood vessel endothelial cells and is implicated in the development and physiological functions of the circulatory system, including the proper formation of blood, vessels, regulation of vascular tone and remodelling of small resistance arteries upon [https://en.wikipedia.org/wiki/Hypertension hypertension]. It is also involved in the homeostasis of the volume of red blood cells.<ref name="Cell Press"/> | ||
| - | Piezo1 mediates cationic non-selective currents. Indeed, monovalent (Na+, K+) and divalent (Ca2+, Mg2+) can flow through Piezo1. | ||
| - | However, Piezo1 is implicated in excitatory channels because cations can enter into the cells which leads to the membrane [https://en.wikipedia.org/wiki/Depolarization depolarisation] or [https://en.wikipedia.org/wiki/Calcium_signaling calcium-dependent signalling pathway] (if Ca2+ enters).<ref name="Adenosine"> DOI 10.3389/fphar.2019.01304 </ref> | ||
| - | When calcium-dependent signalling pathway is activated, NO can be released by endothelial cells and leads to vasodilation but also, some channels can be activated in red blood cells.<ref name="Adenosine"/> | ||
| - | Piezo1 has a wide variety of functions, but we will focus on the vascularisation. | ||
| - | |||
| - | ==='''Vascularisation: detection of shearing forces'''=== | ||
| - | |||
| - | Piezo1 plays a critical role in the formation of blood vessels. Indeed, fluid flow induces a frictional force, and this shear stress activates the Piezo1 channels located in endothelial cells’ membranes. It results in an alignment process, leading to healthy vascular development. The entry of Ca2+ is the key to the process. The shear stress-enhanced Ca2+ entry through Piezo1 channels is coupled with [[calpain]] activation. From this association steams proteolytic cleavage of cytoskeletal [[actin]] and [https://en.wikipedia.org/wiki/Focal_adhesion focal adhesion] proteins, which induces endothelial cell organisation and alignment. | ||
| - | A deficit in Piezo1’s expression can lead to a cobblestone-like appearance of endothelial cells’ organisation, instead of its standard linear appearance. | ||
| - | The subcellular localisation of Piezo1 is also determining. In static conditions, its repartition is even on the membrane, but when a mechanical stimulus arises, Piezo1 accumulates at the cell’s apical. This process characterises endothelial cells’ alignment toward frictional force. | ||
| - | However, Piezo1 is also able to drive endothelial cells migration without shear stress, through endothelial [https://en.wikipedia.org/wiki/Nitric_oxide_synthasenitric oxide synthase], a protein with major roles in vascular biology.<ref name= "vascularisation"> DOI 10.1038/nature13701</ref> | ||
| - | |||
== Structure == | == Structure == | ||
| - | |||
| - | ==='''Gating mechanism'''=== | ||
| - | [[Image:PIEZO.jpg#filehistory | thumb |left|400px| upright=10| '''Membrane deformation and Gating mechanism of Piezo1 before and after activation by an external mechanical force.''' | ||
| - | |||
| - | 1: IH, 2:OH]] | ||
| - | Piezo1 possesses delicate force sensing and mechanotransduction mechanisms. Here, we explain how Piezo1 channels sense and transduce mechanical forces to gate the central ion-conducting pore. | ||
| - | Piezo1 can sense membrane tension through changes in the local curvature of the membrane and the channel opens in response to this change thanks to this structure.<ref name ="Piezo Senses Tension"/> | ||
| - | Indeed, mPiezo trimer is a non-planar conformation inside lipid bilayer, it produces a local dome-shaped deformation of the membrane. In cells, this membrane curvature project towards the cytoplasm and some electrostatic interactions stabilize the trimeric assembly in its curved conformation.<ref name = "nv article"> DOI 10.7554/eLife.33660</ref> | ||
| - | The structure of Piezo1 offers a plausible explanation for the origin of its tension [https://en.wikipedia.org/wiki/Gating_(electrophysiology) gating]. Indeed, if the semi-spherical dome becomes flatter when Piezo1 opens, then the channel membrane system will expand thanks to the flexibility of the blades. | ||
| - | However, because flattening does not constrain the pore to open wide, expansion and pore diameter are decoupled, such that Piezo1 can exhibit its small conductance and cation selectivity, properties that are essential to its function.<ref name ="Piezo Senses Tension"/>,<ref name="Lin" /> | ||
| - | |||
| - | |||
| - | |||
==='''Blade'''=== | ==='''Blade'''=== | ||
| Line 70: | Line 30: | ||
This position and connections of the beam render it an ideal structure for mechanical transmission from the distal THUs to the central ion-conducting pore. The lever-like mechanotransduction apparatus constituted by the beam is possible because of its uneven movement. It displays large motion at the distal beam while subtle movement at the proximal end. It enables Piezo1 channels to effectively convert a large conformational change of the distal blades to a relatively slight opening of the central pore, allowing cation-selective permeation.<ref name="mechanogating"/> | This position and connections of the beam render it an ideal structure for mechanical transmission from the distal THUs to the central ion-conducting pore. The lever-like mechanotransduction apparatus constituted by the beam is possible because of its uneven movement. It displays large motion at the distal beam while subtle movement at the proximal end. It enables Piezo1 channels to effectively convert a large conformational change of the distal blades to a relatively slight opening of the central pore, allowing cation-selective permeation.<ref name="mechanogating"/> | ||
| + | |||
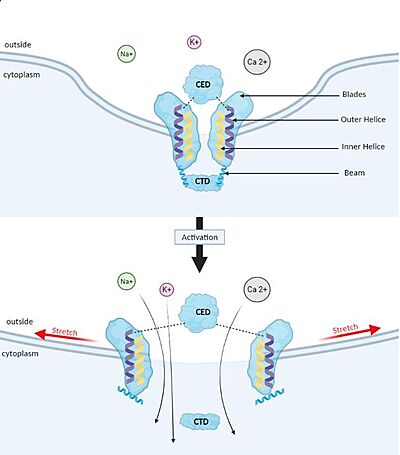
| + | [[Image:Piezo_1_opening_mechanism.JPG#filehistory | thumb |left|400px| upright=10| '''Membrane deformation and Gating mechanism of Piezo1 before and after activation by an external mechanical force.''']] | ||
| + | ==='''Gating mechanism'''=== | ||
| + | Piezo1 possesses delicate force sensing and mechanotransduction mechanisms. Here, we explain how Piezo1 channels sense and transduce mechanical forces to gate the central ion-conducting pore. | ||
| + | Piezo1 can sense membrane tension through changes in the local curvature of the membrane and the channel opens in response to this change thanks to this structure.<ref name ="Piezo Senses Tension"/> | ||
| + | Indeed, mPiezo trimer is a non-planar conformation inside lipid bilayer, it produces a local dome-shaped deformation of the membrane. In cells, this membrane curvature project towards the cytoplasm and some electrostatic interactions stabilize the trimeric assembly in its curved conformation.<ref name = "nv article"> DOI 10.7554/eLife.33660</ref> | ||
| + | The structure of Piezo1 offers a plausible explanation for the origin of its tension [https://en.wikipedia.org/wiki/Gating_(electrophysiology) gating]. Indeed, if the semi-spherical dome becomes flatter when Piezo1 opens, then the channel membrane system will expand thanks to the flexibility of the blades. | ||
| + | However, because flattening does not constrain the pore to open wide, expansion and pore diameter are decoupled, such that Piezo1 can exhibit its small conductance and cation selectivity, properties that are essential to its function.<ref name ="Piezo Senses Tension"/>,<ref name="Lin" /> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Function == | ||
| + | ==='''The different forces perceived by Piezo1'''=== | ||
| + | |||
| + | Piezo1 is a [https://en.wikipedia.org/wiki/Mechanosensitive_channels mechanosensitive channel] which means, it can sense external mechanical forces such as fluid flow-induced shear stress, osmotic stress, and pressure-induced membrane stretch. Moreover, “studies have demonstrated wide expression of the Piezo1 channel that enables many different types of cells to sense a diversity of “outside-in” mechanical forces, including indentation, membrane stretch, shear stress, osmotic stress, ultrasound, and compression”.<ref name= "Adenosine"> DOI 10.3389/fphar.2019.01304</ref> Since Piezo1 channel could also be activated by traction forces, there are two different mechanisms that have been proposed to explain the mechanical activation of Piezo1 channel. These mechanisms are called “force-from-lipids” and “force-from-filaments”.<ref name= "Adenosine" /> | ||
| + | |||
| + | For the “force-from-lipids” mechanism, membrane tension is induced by mechanical forces. This membrane tension leads to a reorganization of lipids within and surrounding the channel proteins. This reorganization of lipids results into membrane lipid-channel protein interactions that induce the channel to open. We can note that a recent study (Lin et al., 2019)<ref name= "Lin"> DOI 10.1038/s41586-019-1499-2 </ref> gave support to this mechanism. | ||
| + | |||
| + | The “force-from-filaments” mechanism proposes that conformational changes occur thanks to interactions between the channel and extracellular matrix or intracellular cytoskeletal proteins resulting in the channel opening.<ref name= "Adenosine"> DOI 10.3389/fphar.2019.01304</ref> | ||
| + | |||
| + | ==='''The wide variety of Piezo1’s functions'''=== | ||
| + | |||
| + | Cells are able to perceive the filling of the stomach or the bladder, blood flow and lungs inflation. | ||
| + | |||
| + | Piezo1 is a sensor of mechanical forces in [https://en.wikipedia.org/wiki/Endothelium endothelial], urothelial and renal epithelial cells. For instance, Piezo1 is involved in shear stress sensing in blood vessel endothelial cells and is implicated in the development and physiological functions of the circulatory system, including the proper formation of blood, vessels, regulation of vascular tone and remodelling of small resistance arteries upon [https://en.wikipedia.org/wiki/Hypertension hypertension]. It is also involved in the homeostasis of the volume of red blood cells.<ref name="Cell Press"/> | ||
| + | Piezo1 mediates cationic non-selective currents. Indeed, monovalent (Na+, K+) and divalent (Ca2+, Mg2+) can flow through Piezo1. | ||
| + | However, Piezo1 is implicated in excitatory channels because cations can enter into the cells which leads to the membrane [https://en.wikipedia.org/wiki/Depolarization depolarisation] or [https://en.wikipedia.org/wiki/Calcium_signaling calcium-dependent signalling pathway] (if Ca2+ enters).<ref name="Adenosine"> DOI 10.3389/fphar.2019.01304 </ref> | ||
| + | When calcium-dependent signalling pathway is activated, NO can be released by endothelial cells and leads to vasodilation but also, some channels can be activated in red blood cells.<ref name="Adenosine"/> | ||
| + | Piezo1 has a wide variety of functions, but we will focus on the vascularisation. | ||
| + | |||
| + | ==='''Vascularisation: detection of shearing forces'''=== | ||
| + | |||
| + | Piezo1 plays a critical role in the formation of blood vessels. Indeed, fluid flow induces a frictional force, and this shear stress activates the Piezo1 channels located in endothelial cells’ membranes. It results in an alignment process, leading to healthy vascular development. The entry of Ca2+ is the key to the process. The shear stress-enhanced Ca2+ entry through Piezo1 channels is coupled with [[calpain]] activation. From this association steams proteolytic cleavage of cytoskeletal [[actin]] and [https://en.wikipedia.org/wiki/Focal_adhesion focal adhesion] proteins, which induces endothelial cell organisation and alignment. | ||
| + | A deficit in Piezo1’s expression can lead to a cobblestone-like appearance of endothelial cells’ organisation, instead of its standard linear appearance. | ||
| + | The subcellular localisation of Piezo1 is also determining. In static conditions, its repartition is even on the membrane, but when a mechanical stimulus arises, Piezo1 accumulates at the cell’s apical. This process characterises endothelial cells’ alignment toward frictional force. | ||
| + | However, Piezo1 is also able to drive endothelial cells migration without shear stress, through endothelial [https://en.wikipedia.org/wiki/Nitric_oxide_synthasenitric oxide synthase], a protein with major roles in vascular biology.<ref name= "vascularisation"> DOI 10.1038/nature13701</ref> | ||
== Disease == | == Disease == | ||
Revision as of 12:55, 14 January 2022
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Zhang M, Xiao B. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron. 2016 Mar 16;89(6):1248-1263. doi: 10.1016/j.neuron.2016.01.046. Epub 2016, Feb 25. PMID:26924440 doi:http://dx.doi.org/10.1016/j.neuron.2016.01.046
- ↑ 2.0 2.1 Parpaite T, Coste B. Piezo channels. Curr Biol. 2017 Apr 3;27(7):R250-R252. doi: 10.1016/j.cub.2017.01.048. PMID:28376327 doi:http://dx.doi.org/10.1016/j.cub.2017.01.048
- ↑ 3.0 3.1 3.2 Zhou, Z. (2019). Structural Analysis of Piezo1 Ion Channel Reveals the Relationship between Amino Acid Sequence Mutations and Human Diseases. 139–155. DOI 10.4236/jbm.2019.712012
- ↑ 4.0 4.1 4.2 4.3 4.4 Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018 Feb 22;554(7693):487-492. doi: 10.1038/nature25743. Epub 2018 Jan, 22. PMID:29469092 doi:http://dx.doi.org/10.1038/nature25743
- ↑ 5.0 5.1 5.2 5.3 Liang X, Howard J. Structural Biology: Piezo Senses Tension through Curvature. Curr Biol. 2018 Apr 23;28(8):R357-R359. doi: 10.1016/j.cub.2018.02.078. PMID:29689211 doi:http://dx.doi.org/10.1016/j.cub.2018.02.078
- ↑ 6.0 6.1 Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017 Dec 12;6. pii: 33660. doi: 10.7554/eLife.33660. PMID:29231809 doi:http://dx.doi.org/10.7554/eLife.33660
- ↑ 7.0 7.1 7.2 Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015 Nov 5;527(7576):64-9. doi: 10.1038/nature15247. Epub 2015 Sep 21. PMID:26390154 doi:http://dx.doi.org/10.1038/nature15247
- ↑ 8.0 8.1 Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2017 Dec 20. pii: nature25453. doi: 10.1038/nature25453. PMID:29261642 doi:http://dx.doi.org/10.1038/nature25453
- ↑ 9.0 9.1 Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019 Sep;573(7773):230-234. doi: 10.1038/s41586-019-1499-2. Epub 2019 Aug, 21. PMID:31435018 doi:http://dx.doi.org/10.1038/s41586-019-1499-2
- ↑ 10.0 10.1 10.2 10.3 10.4 Wei L, Mousawi F, Li D, Roger S, Li J, Yang X, Jiang LH. Adenosine Triphosphate Release and P2 Receptor Signaling in Piezo1 Channel-Dependent Mechanoregulation. Front Pharmacol. 2019 Nov 6;10:1304. doi: 10.3389/fphar.2019.01304. eCollection, 2019. PMID:31780935 doi:http://dx.doi.org/10.3389/fphar.2019.01304
- ↑ Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad KR, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature. 2014 Nov 13;515(7526):279-82. doi: 10.1038/nature13701. Epub 2014 Aug 10. PMID:25119035 doi:http://dx.doi.org/10.1038/nature13701
- ↑ 12.0 12.1 Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garcon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. PMID:23695678 doi:http://dx.doi.org/10.1038/ncomms2899
- ↑ Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE, Narayan R, Montanaro D, D'Armiento M, Vetro A, Limongelli I, Zuffardi O, Glader BE, Schrier SL, Brugnara C, Stewart GW, Delaunay J, Iolascon A. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013 May 9;121(19):3925-35, S1-12. doi: 10.1182/blood-2013-02-482489. Epub, 2013 Mar 11. PMID:23479567 doi:http://dx.doi.org/10.1182/blood-2013-02-482489