We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1647
From Proteopedia
(Difference between revisions)
| Line 44: | Line 44: | ||
The secondary structure of the protein allows it to bind with the DNA : the T-box domain consists of several repeats of <scene name='86/868180/Helix_and_strand/1'>β-strands and α-helix</scene> and is involved in both dimerization and DNA binding. | The secondary structure of the protein allows it to bind with the DNA : the T-box domain consists of several repeats of <scene name='86/868180/Helix_and_strand/1'>β-strands and α-helix</scene> and is involved in both dimerization and DNA binding. | ||
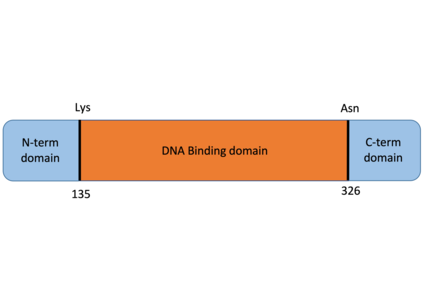
| - | Thanks to some [https://en.wikipedia.org/wiki/Post-translational_modification post-translational modifications] of the protein’s residues, the transcription factor TBX21 can bind with DNA between the residues <scene name='86/868180/Lys135/2'>LYS 135</scene> and <scene name='86/868180/Asn_326/4'>ASN 326</scene> and some proteins. Firstly, the ubiquitination of the residue K 313 allows TBX21 to bind with the DNA sequence. Lys-313 was lately found as a key site required for T-bet to interact with the [https://proteopedia.org/wiki/index.php/6f1e IFN-γ] gene promoter and to assure phosphorylation at Thr-302 <ref name="Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet">DOI 10.1073/pnas.0409512102</ref>. Secondly, the phosphorylation of some residues allows TBX21 to interact with several proteins. For example, phosphorylation of T 302 allows TBX21 to interact with [https://proteopedia.org/wiki/index.php/2o93 NFAT], the one of Y 304 with [https://proteopedia.org/wiki/index.php/1co1 RUNX1], that of S 508 with [https://proteopedia.org/wiki/index.php/2i9t NF-кB p65] and finally the one of Y525 allows the interaction with <scene name='86/868180/Dna_binding_by_gata_transcript/2'>GATA-3</scene><ref name="The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes">DOI 10.1073/pnas.0909357106</ref> | + | Thanks to some [https://en.wikipedia.org/wiki/Post-translational_modification post-translational modifications] of the protein’s residues, the transcription factor TBX21 can bind with DNA between the residues <scene name='86/868180/Lys135/2'>LYS 135</scene> and <scene name='86/868180/Asn_326/4'>ASN 326</scene> and some proteins. Firstly, the ubiquitination of the residue K 313 allows TBX21 to bind with the DNA sequence. Lys-313 was lately found as a key site required for T-bet to interact with the [https://proteopedia.org/wiki/index.php/6f1e IFN-γ] gene promoter and to assure phosphorylation at Thr-302 <ref name="Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet">DOI 10.1073/pnas.0409512102</ref>. Secondly, the phosphorylation of some residues allows TBX21 to interact with several proteins. For example, phosphorylation of T 302 allows TBX21 to interact with [https://proteopedia.org/wiki/index.php/2o93 NFAT], the one of Y 304 with [https://proteopedia.org/wiki/index.php/1co1 RUNX1], that of S 508 with [https://proteopedia.org/wiki/index.php/2i9t NF-кB p65] and finally the one of Y525 allows the interaction with <scene name='86/868180/Dna_binding_by_gata_transcript/2'>GATA-3</scene> <ref name="The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes">DOI 10.1073/pnas.0909357106</ref>. |
| Line 50: | Line 50: | ||
=== Regulation of Th cells differentiation by TBX21 === | === Regulation of Th cells differentiation by TBX21 === | ||
The transcription factor T-bet directs Th1 cell differentiation. The molecular mechanisms that underlie this lineage-specific gene regulation are not completely understood but several hypotheses have already been made on the action's mechanism of T-bet. <ref name="NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression">DOI 10.4049/jimmunol.1203403</ref> | The transcription factor T-bet directs Th1 cell differentiation. The molecular mechanisms that underlie this lineage-specific gene regulation are not completely understood but several hypotheses have already been made on the action's mechanism of T-bet. <ref name="NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression">DOI 10.4049/jimmunol.1203403</ref> | ||
| - | We know that T-bet initiates Th1 lineage development from naive Thp cells by activating Th1 genetics and repressing the opposing Th2 programs. Th1 cells stimulate cellular immune response while Th2 stimulates humoral immune response and induces antibody production | + | We know that T-bet initiates Th1 lineage development from naive Thp cells by activating Th1 genetics and repressing the opposing Th2 programs. Th1 cells stimulate cellular immune response while Th2 stimulates humoral immune response and induces antibody production <ref name="The Transcription Factor T-Bet Is Required for Optimal Type I Follicular Helper T Cell Maintenance During Acute Viral Infection">DOI 10.3389/fimmu.2019.00606</ref>. |
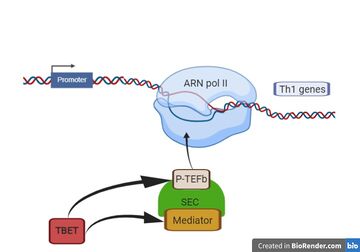
[[Image:TBET.jpg|thumb|upright=2 |T-bet action in the formation of SEC]] | [[Image:TBET.jpg|thumb|upright=2 |T-bet action in the formation of SEC]] | ||
| - | Here, we show that T-bet acts through enhancers to allow the recruitment of [[Mediator]] and [https://proteopedia.org/wiki/index.php/3mi9 P-TEFb] in the formation of the super elongation complex (SEC). Th1 genes are occupied by [[RNA Polymerase II]] in Thp cells, while T-bet-mediated recruitment of P-TEFb and mediator. The recruitment of P-TEFb and mediator activates transcriptional elongation and giving place to increased differentiation of Thp into Th1 | + | Here, we show that T-bet acts through enhancers to allow the recruitment of [[Mediator]] and [https://proteopedia.org/wiki/index.php/3mi9 P-TEFb] in the formation of the super elongation complex (SEC). Th1 genes are occupied by [[RNA Polymerase II]] in Thp cells, while T-bet-mediated recruitment of P-TEFb and mediator. The recruitment of P-TEFb and mediator activates transcriptional elongation and giving place to increased differentiation of Thp into Th1 <ref name="T-bet Activates Th1 Genes through Mediator and the Super Elongation Complex">DOI 10.1016/j.celrep.2016.05.054</ref>. |
T-bet can also regulate Th1 cell differentiation by directly initiating gamma interferon (IFN-γ) transcription <ref name="The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation.">DOI 10.1038/ni.1731</ref>and by suppressing Th2-specific transcription factor [https://proteopedia.org/wiki/index.php/3dfx GATA-3]<ref name="A novel transcription factor, T-bet, directs Th1 lineage commitment"> Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, C. G., & Glimcher, L. H. (2000). A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell, 100(6), 655–669. https://doi.org/10.1016/s0092-8674(00)80702-3</ref>. The T-bet induced expression of IFN-γ derives Th precursor cells to differentiate into Th1 effector cells. | T-bet can also regulate Th1 cell differentiation by directly initiating gamma interferon (IFN-γ) transcription <ref name="The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation.">DOI 10.1038/ni.1731</ref>and by suppressing Th2-specific transcription factor [https://proteopedia.org/wiki/index.php/3dfx GATA-3]<ref name="A novel transcription factor, T-bet, directs Th1 lineage commitment"> Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, C. G., & Glimcher, L. H. (2000). A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell, 100(6), 655–669. https://doi.org/10.1016/s0092-8674(00)80702-3</ref>. The T-bet induced expression of IFN-γ derives Th precursor cells to differentiate into Th1 effector cells. | ||
| Line 65: | Line 65: | ||
=== TBX21 as an antiasthmatic regulator === | === TBX21 as an antiasthmatic regulator === | ||
| - | Asthma remains one of the commonest chronic inflammatory diseases and has a major impact on the life of sufferers. It is associated with allergy mediated by [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE antibodies]. T-bet was found associated with many immune-mediated diseases such as asthma | + | Asthma remains one of the commonest chronic inflammatory diseases and has a major impact on the life of sufferers. It is associated with allergy mediated by [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE antibodies]. T-bet was found associated with many immune-mediated diseases such as asthma <ref name="Asthma: T-bet--a master controller?">Douglas.S.Robinson and Clare M Lloyd. Asthma: T-bet - A master controller ? Volume 12, Issue 9, PR322-R324, April 30, (2002) https://doi.org/10.1016/S0960-9822(02)00830-8</ref>. |
| - | Th1, Th2 and Th17 cells are the main cells involved in the pathophysiology of asthma <ref name="Effect of ginger extract on expression of GATA3, T-bet and ROR-γt in peripheral blood mononuclear cells of patients with Allergic Asthma">DOI 10.1016/j.aller.2018.12.003</ref>. In asthmatic airways, Th2 cells are activated and release several cytokines <ref name="The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells">DOI 10.1155/2014/589672</ref> that regulate IgE production and inflammatory cell recruitment, such as eosinophils. Th2 cells and GATA-3 play an important role in allergic inflammation and asthma, and induce IgE production, which can be the cause of severe forms of asthma. The asthmatic patients present high levels of total IgE. On the contrary, the T-bet gene expression and Th1 pattern, along with the IFN- γ production, are usually associated with non-allergic asthmatics and healthy subjects | + | Th1, Th2 and Th17 cells are the main cells involved in the pathophysiology of asthma <ref name="Effect of ginger extract on expression of GATA3, T-bet and ROR-γt in peripheral blood mononuclear cells of patients with Allergic Asthma">DOI 10.1016/j.aller.2018.12.003</ref>. In asthmatic airways, Th2 cells are activated and release several cytokines <ref name="The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells">DOI 10.1155/2014/589672</ref> that regulate IgE production and inflammatory cell recruitment, such as eosinophils. Th2 cells and GATA-3 play an important role in allergic inflammation and asthma, and induce IgE production, which can be the cause of severe forms of asthma. The asthmatic patients present high levels of total IgE. On the contrary, the T-bet gene expression and Th1 pattern, along with the IFN- γ production, are usually associated with non-allergic asthmatics and healthy subjects <ref name="Asthma: T-bet--a master controller?">DOI 10.1016/s0960-9822(02)00830-8</ref>. Also, some research has found that airway reactivity is moderated by the use of corticosteroids in asthma patients and with the use of it the TBX21 variant increases Th1 and decreases Th2 cytokine expression. So TBX21 may be determinant in the therapy of asthma with inhaled corticosteroids <ref name="TBX21: A functional variant predicts improvement in asthma with the use of inhaled corticosteroids">DOI 10.1073/pnas.0408532102</ref>. |
In T-bet structure, ubiquitination takes place at LYS 313. It has an impact on the stability of the protein and leads to the degradation of the protein by the proteosome. Some research found the role of deubiquitinases involved in T-bet stability and function. As a deubiquitinase, USP10 belongs to the ubiquitin-specificprotease family of cysteine proteases. Cysteine 424 site on [https://en.wikipedia.org/wiki/USP10 USP10] is crucial for its hydrolase activity. Results have shown that USP10 could interact with T-bet and stabilize it via interaction between Lysine 313 of T-bet and Cysteine 424 of USP10. Deubiquitination inhibits its degradation by the proteosome and enhance the secretion of IFN- γ. | In T-bet structure, ubiquitination takes place at LYS 313. It has an impact on the stability of the protein and leads to the degradation of the protein by the proteosome. Some research found the role of deubiquitinases involved in T-bet stability and function. As a deubiquitinase, USP10 belongs to the ubiquitin-specificprotease family of cysteine proteases. Cysteine 424 site on [https://en.wikipedia.org/wiki/USP10 USP10] is crucial for its hydrolase activity. Results have shown that USP10 could interact with T-bet and stabilize it via interaction between Lysine 313 of T-bet and Cysteine 424 of USP10. Deubiquitination inhibits its degradation by the proteosome and enhance the secretion of IFN- γ. | ||
| - | Researchers believe that the USP10-dependent T-bet deubiquitination and stabilization can regulate antigen induced immune disorder especially in Th1 specific inflammation. Thus, appropriate decreasing USP10 level may contribute to the T-bet degradation and inflammation attenuation | + | Researchers believe that the USP10-dependent T-bet deubiquitination and stabilization can regulate antigen induced immune disorder especially in Th1 specific inflammation. Thus, appropriate decreasing USP10 level may contribute to the T-bet degradation and inflammation attenuation <ref name="Deubiquitination and stabilization of T-bet by USP10">DOI 10.1016/j.bbrc.2014.05.037</ref>. |
| - | Over the past few years, it has been shown by researchers that the polymorphism of TBX21 could act on nasal polyps and aspirin intolerance too. Patients that cumulate aspirin-intolerance and asthma suffer from bronchoconstriction when using aspririn. This bronchoconstriction is due to the production of cysteinyl leukotrienes, a family of inflammatory mediators, triggered by aspirin intake | + | Over the past few years, it has been shown by researchers that the polymorphism of TBX21 could act on nasal polyps and aspirin intolerance too. Patients that cumulate aspirin-intolerance and asthma suffer from bronchoconstriction when using aspririn. This bronchoconstriction is due to the production of cysteinyl leukotrienes, a family of inflammatory mediators, triggered by aspirin intake <ref>PMID: 512268</ref>. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 16:42, 14 January 2022
| |||||||||||
References
- ↑ TBX21/T-Bet polyclonal antibody : https://www.thermofisher.com/antibody/product/TBX21-T-bet-Antibody-Polyclonal/13700-1-AP
- ↑ TBX21-GeneCards : TBX21 - T-box Transcription factor 21 :https://www.genecards.org/cgi-bin/carddisp.pl?gene=TBX21
- ↑ Mehta DS, Wurster AL, Weinmann AS, Grusby MJ. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc Natl Acad Sci U S A. 2005 Feb 8;102(6):2016-21. doi:, 10.1073/pnas.0409512102. Epub 2005 Jan 31. PMID:15684054 doi:http://dx.doi.org/10.1073/pnas.0409512102
- ↑ Jang EJ, Park HR, Hong JH, Hwang ES. Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet. J Immunol. 2013 Jun 1;190(11):5764-70. doi: 10.4049/jimmunol.1203403. Epub 2013, Apr 24. PMID:23616576 doi:http://dx.doi.org/10.4049/jimmunol.1203403
- ↑ Wang P, Wang Y, Xie L, Xiao M, Wu J, Xu L, Bai Q, Hao Y, Huang Q, Chen X, He R, Li B, Yang S, Chen Y, Wu Y, Ye L. The Transcription Factor T-Bet Is Required for Optimal Type I Follicular Helper T Cell Maintenance During Acute Viral Infection. Front Immunol. 2019 Mar 29;10:606. doi: 10.3389/fimmu.2019.00606. eCollection, 2019. PMID:30984183 doi:http://dx.doi.org/10.3389/fimmu.2019.00606
- ↑ Hertweck A, Evans CM, Eskandarpour M, Lau JC, Oleinika K, Jackson I, Kelly A, Ambrose J, Adamson P, Cousins DJ, Lavender P, Calder VL, Lord GM, Jenner RG. T-bet Activates Th1 Genes through Mediator and the Super Elongation Complex. Cell Rep. 2016 Jun 21;15(12):2756-70. doi: 10.1016/j.celrep.2016.05.054. Epub, 2016 Jun 9. PMID:27292648 doi:http://dx.doi.org/10.1016/j.celrep.2016.05.054
- ↑ Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009 Jun;10(6):595-602. doi: 10.1038/ni.1731. Epub 2009 May 3. PMID:19412181 doi:http://dx.doi.org/10.1038/ni.1731
- ↑ Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, C. G., & Glimcher, L. H. (2000). A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell, 100(6), 655–669. https://doi.org/10.1016/s0092-8674(00)80702-3
- ↑ Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011 Jan;12(1):96-104. doi: 10.1038/ni.1969. Epub 2010 Dec 12. PMID:21151104 doi:http://dx.doi.org/10.1038/ni.1969
- ↑ 11.0 11.1 Douglas.S.Robinson and Clare M Lloyd. Asthma: T-bet - A master controller ? Volume 12, Issue 9, PR322-R324, April 30, (2002) https://doi.org/10.1016/S0960-9822(02)00830-8
- ↑ Kardan M, Rafiei A, Ghaffari J, Valadan R, Morsaljahan Z, Haj-Ghorbani ST. Effect of ginger extract on expression of GATA3, T-bet and ROR-gammat in peripheral blood mononuclear cells of patients with Allergic Asthma. Allergol Immunopathol (Madr). 2019 Jul - Aug;47(4):378-385. doi:, 10.1016/j.aller.2018.12.003. Epub 2019 Feb 10. PMID:30745246 doi:http://dx.doi.org/10.1016/j.aller.2018.12.003
- ↑ Oh S, Hwang ES. The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res. 2014;2014:589672. doi: 10.1155/2014/589672. Epub 2014 May 13. PMID:24901011 doi:http://dx.doi.org/10.1155/2014/589672
- ↑ Tantisira KG, Hwang ES, Raby BA, Silverman ES, Lake SL, Richter BG, Peng SL, Drazen JM, Glimcher LH, Weiss ST. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18099-104. doi:, 10.1073/pnas.0408532102. Epub 2004 Dec 16. PMID:15604153 doi:http://dx.doi.org/10.1073/pnas.0408532102
- ↑ Pan L, Chen Z, Wang L, Chen C, Li D, Wan H, Li B, Shi G. Deubiquitination and stabilization of T-bet by USP10. Biochem Biophys Res Commun. 2014 Jul 4;449(3):289-94. doi:, 10.1016/j.bbrc.2014.05.037. Epub 2014 May 17. PMID:24845384 doi:http://dx.doi.org/10.1016/j.bbrc.2014.05.037
- ↑ Spector SL, Wangaard CH, Farr RS. Aspirin and concomitant idiosyncrasies in adult asthmatic patients. J Allergy Clin Immunol. 1979 Dec;64(6 Pt 1):500-6. doi:, 10.1016/0091-6749(79)90059-9. PMID:512268 doi:http://dx.doi.org/10.1016/0091-6749(79)90059-9