We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1653
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<references/><StructureSection load='5z10' size='350' side='right' caption='Structure of the mechanosensitive Piezo1 channel 1 from [http://www.rcsb.org/structure/5Z10 PBD]' scene=''> | <references/><StructureSection load='5z10' size='350' side='right' caption='Structure of the mechanosensitive Piezo1 channel 1 from [http://www.rcsb.org/structure/5Z10 PBD]' scene=''> | ||
| - | Piezo1 proteins constitute a family of excitatory [[ion channels]] directly gated by mechanical forces. Piezo1 is functionally conserved and very important because all living organisms are subjected to mechanical forces from their environment for instance [https://en.wikipedia.org/wiki/Proprioception proprioception], [https://en.wikipedia.org/wiki/Osmoregulation osmoregulation], vascular tone, blood flow regulation, muscle [https://en.wikipedia.org/wiki/Homeostasis homeostasis], flow sensing in kidney, bladder and lungs.<ref name="Ion Permeation"> DOI 10.1016/j.neuron.2016.01.046 | + | Piezo1 proteins constitute a family of excitatory [[ion channels]] directly gated by mechanical forces. Piezo1 is functionally conserved and very important because all living organisms are subjected to mechanical forces from their environment for instance [https://en.wikipedia.org/wiki/Proprioception proprioception], [https://en.wikipedia.org/wiki/Osmoregulation osmoregulation], vascular tone, blood flow regulation, muscle [https://en.wikipedia.org/wiki/Homeostasis homeostasis], flow sensing in kidney, bladder and lungs.<ref name="Ion Permeation"> DOI 10.1016/j.neuron.2016.01.046 </ref> |
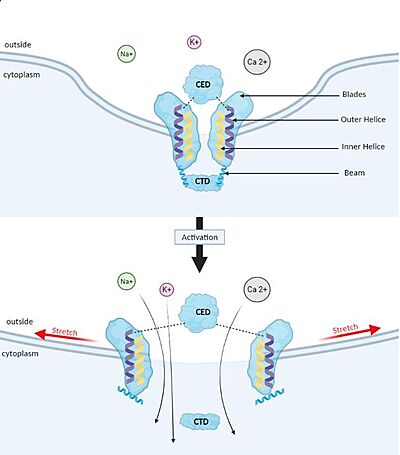
== Structure == | == Structure == | ||
| Line 56: | Line 56: | ||
Cells are able to perceive the filling of the stomach or the bladder, blood flow and lungs inflation. | Cells are able to perceive the filling of the stomach or the bladder, blood flow and lungs inflation. | ||
| - | Piezo1 is a sensor of mechanical forces in [https://en.wikipedia.org/wiki/Endothelium endothelial], urothelial and renal epithelial cells. For instance, Piezo1 is involved in shear stress sensing in blood vessel endothelial cells and is implicated in the development and physiological functions of the circulatory system, including the proper formation of blood, vessels, regulation of vascular tone and remodelling of small resistance arteries upon [https://en.wikipedia.org/wiki/Hypertension hypertension]. It is also involved in the homeostasis of the volume of red blood cells. | + | Piezo1 is a sensor of mechanical forces in [https://en.wikipedia.org/wiki/Endothelium endothelial], urothelial and renal epithelial cells. For instance, Piezo1 is involved in shear stress sensing in blood vessel endothelial cells and is implicated in the development and physiological functions of the circulatory system, including the proper formation of blood, vessels, regulation of vascular tone and remodelling of small resistance arteries upon [https://en.wikipedia.org/wiki/Hypertension hypertension]. It is also involved in the homeostasis of the volume of red blood cells. |
Piezo1 mediates cationic non-selective currents. Indeed, monovalent (Na+, K+) and divalent (Ca2+, Mg2+) can flow through Piezo1. | Piezo1 mediates cationic non-selective currents. Indeed, monovalent (Na+, K+) and divalent (Ca2+, Mg2+) can flow through Piezo1. | ||
However, Piezo1 is implicated in excitatory channels because cations can enter into the cells which leads to the membrane [https://en.wikipedia.org/wiki/Depolarization depolarisation] or [https://en.wikipedia.org/wiki/Calcium_signaling calcium-dependent signalling pathway] (if Ca2+ enters).<ref name="Adenosine"> DOI 10.3389/fphar.2019.01304 </ref> | However, Piezo1 is implicated in excitatory channels because cations can enter into the cells which leads to the membrane [https://en.wikipedia.org/wiki/Depolarization depolarisation] or [https://en.wikipedia.org/wiki/Calcium_signaling calcium-dependent signalling pathway] (if Ca2+ enters).<ref name="Adenosine"> DOI 10.3389/fphar.2019.01304 </ref> | ||
Revision as of 16:44, 16 January 2022
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Zhang M, Xiao B. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron. 2016 Mar 16;89(6):1248-1263. doi: 10.1016/j.neuron.2016.01.046. Epub 2016, Feb 25. PMID:26924440 doi:http://dx.doi.org/10.1016/j.neuron.2016.01.046
- ↑ 2.0 2.1 2.2 Zhou, Z. (2019). Structural Analysis of Piezo1 Ion Channel Reveals the Relationship between Amino Acid Sequence Mutations and Human Diseases. 139–155. DOI 10.4236/jbm.2019.712012
- ↑ 3.0 3.1 3.2 3.3 3.4 Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018 Feb 22;554(7693):487-492. doi: 10.1038/nature25743. Epub 2018 Jan, 22. PMID:29469092 doi:http://dx.doi.org/10.1038/nature25743
- ↑ 4.0 4.1 4.2 4.3 Liang X, Howard J. Structural Biology: Piezo Senses Tension through Curvature. Curr Biol. 2018 Apr 23;28(8):R357-R359. doi: 10.1016/j.cub.2018.02.078. PMID:29689211 doi:http://dx.doi.org/10.1016/j.cub.2018.02.078
- ↑ 5.0 5.1 Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017 Dec 12;6. pii: 33660. doi: 10.7554/eLife.33660. PMID:29231809 doi:http://dx.doi.org/10.7554/eLife.33660
- ↑ 6.0 6.1 6.2 Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015 Nov 5;527(7576):64-9. doi: 10.1038/nature15247. Epub 2015 Sep 21. PMID:26390154 doi:http://dx.doi.org/10.1038/nature15247
- ↑ 7.0 7.1 Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2017 Dec 20. pii: nature25453. doi: 10.1038/nature25453. PMID:29261642 doi:http://dx.doi.org/10.1038/nature25453
- ↑ 8.0 8.1 Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019 Sep;573(7773):230-234. doi: 10.1038/s41586-019-1499-2. Epub 2019 Aug, 21. PMID:31435018 doi:http://dx.doi.org/10.1038/s41586-019-1499-2
- ↑ 9.0 9.1 Chong J, De Vecchis D, Hyman AJ, Povstyan OV, Ludlow MJ, Shi J, Beech DJ, Kalli AC. Modeling of full-length Piezo1 suggests importance of the proximal N-terminus for dome structure. Biophys J. 2021 Apr 20;120(8):1343-1356. doi: 10.1016/j.bpj.2021.02.003. Epub, 2021 Feb 12. PMID:33582137 doi:http://dx.doi.org/10.1016/j.bpj.2021.02.003
- ↑ 10.0 10.1 10.2 10.3 10.4 Wei L, Mousawi F, Li D, Roger S, Li J, Yang X, Jiang LH. Adenosine Triphosphate Release and P2 Receptor Signaling in Piezo1 Channel-Dependent Mechanoregulation. Front Pharmacol. 2019 Nov 6;10:1304. doi: 10.3389/fphar.2019.01304. eCollection, 2019. PMID:31780935 doi:http://dx.doi.org/10.3389/fphar.2019.01304
- ↑ Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad KR, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature. 2014 Nov 13;515(7526):279-82. doi: 10.1038/nature13701. Epub 2014 Aug 10. PMID:25119035 doi:http://dx.doi.org/10.1038/nature13701
- ↑ Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garcon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. PMID:23695678 doi:http://dx.doi.org/10.1038/ncomms2899
- ↑ Yamaguchi Y, Allegrini B, Rapetti-Mauss R, Picard V, Garcon L, Kohl P, Soriani O, Peyronnet R, Guizouarn H. Hereditary Xerocytosis: Differential Behavior of PIEZO1 Mutations in the N-Terminal Extracellular Domain Between Red Blood Cells and HEK Cells. Front Physiol. 2021 Oct 18;12:736585. doi: 10.3389/fphys.2021.736585. eCollection, 2021. PMID:34737711 doi:http://dx.doi.org/10.3389/fphys.2021.736585
- ↑ Alper SL. Genetic Diseases of PIEZO1 and PIEZO2 Dysfunction. Curr Top Membr. 2017;79:97-134. doi: 10.1016/bs.ctm.2017.01.001. Epub 2017 Feb, 28. PMID:28728825 doi:http://dx.doi.org/10.1016/bs.ctm.2017.01.001
- ↑ Shinge SAU, Zhang D, Achu Muluh T, Nie Y, Yu F. Mechanosensitive Piezo1 Channel Evoked-Mechanical Signals in Atherosclerosis. J Inflamm Res. 2021 Jul 27;14:3621-3636. doi: 10.2147/JIR.S319789. eCollection, 2021. PMID:34349540 doi:http://dx.doi.org/10.2147/JIR.S319789
- ↑ Velasco-Estevez M, Gadalla KKE, Linan-Barba N, Cobb S, Dev KK, Sheridan GK. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia. 2020 Feb;68(2):356-375. doi: 10.1002/glia.23722. Epub 2019 Oct 9. PMID:31596529 doi:http://dx.doi.org/10.1002/glia.23722
- ↑ Liu S, Pan X, Cheng W, Deng B, He Y, Zhang L, Ning Y, Li J. Tubeimoside I Antagonizes Yoda1-Evoked Piezo1 Channel Activation. Front Pharmacol. 2020 May 25;11:768. doi: 10.3389/fphar.2020.00768. eCollection, 2020. PMID:32523536 doi:http://dx.doi.org/10.3389/fphar.2020.00768