We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1724
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
===Overview=== | ===Overview=== | ||
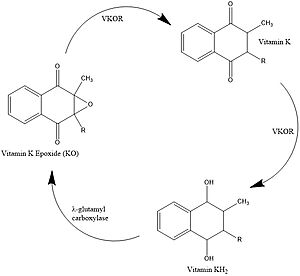
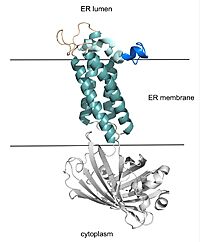
| - | The catalytic cycle shows how vitamin K epoxide reductase structurally transforms from an open wild type conformation to having several different types of substrates within its binding pocket. The first step of the catalytic cycle of shown to the right is the wild type open conformation, labeled I. This step is characterized by an open cap domain with a disulfide bond (43-51) and a second disulfide bond (132-135) in the alpha helices. As shown this step can be characterized as closed when warfarin sits within the binding pocket without the disulfide bonds changing so that the cap domain does not actually close. This step is considered closed because vitamin K would not be able to enter the binding pocket in any of its forms. The second step of the catalytic cycle is a closed conformation labeled II. This step is characterized by a disulfide bond between the cap domain and alpha helices, with both containing an SH group. Warfarin can sit within this structure without disrupting and of these sulfur groups. The next step of the cycle, labeled III, is slightly different because KOH or KH (depending on the step of the vitamin K cycle) binds to the cysteine 135 within the alpha helices. This is also a closed structure. Lastly, structure IV of the catalytic cycle is also a closed structure. The major difference is the orientation of the disulfide and cysteine interactions. | + | The catalytic cycle shows how vitamin K epoxide reductase structurally transforms from an open wild type conformation to having several different types of substrates within its binding pocket. The first step of the catalytic cycle of shown to the right is the <scene name='90/904329/Cat_cycle_i/1'>wild type open conformation</scene>, labeled I. This step is characterized by an open cap domain with a disulfide bond (43-51) and a second disulfide bond (132-135) in the alpha helices. As shown this step can be characterized as closed when warfarin sits within the binding pocket without the disulfide bonds changing so that the cap domain does not actually close. This step is considered closed because vitamin K would not be able to enter the binding pocket in any of its forms. The second step of the catalytic cycle is a closed conformation labeled II. This step is characterized by a disulfide bond between the cap domain and alpha helices, with both containing an SH group. Warfarin can sit within this structure without disrupting and of these sulfur groups. The next step of the cycle, labeled III, is slightly different because KOH or KH (depending on the step of the vitamin K cycle) binds to the cysteine 135 within the alpha helices. This is also a closed structure. Lastly, structure IV of the catalytic cycle is also a closed structure. The major difference is the orientation of the disulfide and cysteine interactions. |
===Catalytic Cysteines=== | ===Catalytic Cysteines=== | ||
Revision as of 00:03, 29 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
Student Contributors
Izabella Jordan, Emma Varness