Sandbox Reserved 1700
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
=== G-Protein === | === G-Protein === | ||
| - | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of alpha, beta, and gamma subunits that interact with membrane receptor proteins. G-proteins are responsible transmitting extracellular signals into the cell upon activation. This activation happens when the alpha subunit of the G-protein binds GTP instead of GDP, and then | + | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of alpha, beta, and gamma subunits that interact with membrane receptor proteins. G-proteins are responsible transmitting extracellular signals into the cell upon activation. This activation happens when the alpha subunit of the G-protein binds GTP instead of GDP, and then disassociates from the rest of the protein, initiating the intracellular signaling cascade. There are different families of G-alpha subunits, Gαi, Gαs, Gα12/13, and Gαq <ref name="Kamato">PMID: 26664886</ref>. MRGPRX2 has been found to bind both Gαi and Gαq subunits with relatively no major structural changes between the two despite slightly different amino acids present <ref name= "Cao" /> <ref name= "Yang" />. Throughout this page, MGPRX2 is always shown with Gq. Figure 2a shows the overlay of MGPRX2 with either the Gq or Gi alpha subunit. Figure 2b shows the specific residues involved in the interface between the membrane receptor and G protein. The major difference between the two comes from one amino acid difference (valine on Gq versus phenylalanine on Gi) that pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. This is the only major structural difference between Gq and Gi subunits. |
| + | |||
| + | [[Image:Image:Gq and gi overlay.png|500px|center|thumb|Figure 2a. Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow). Figure 2b. Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.]] | ||
| + | |||
=== Novel Characteristics === | === Novel Characteristics === | ||
| Line 64: | Line 67: | ||
MRGPRX2 has an <scene name='90/904306/Erc_motif_3/1'>ERC Motif</scene> rather than the typically [https://proteopedia.org/wiki/index.php/A_Physical_Model_of_the_%CE%B22-Adrenergic_Receptor#conserved%20DRY%20motif conserved E/DRY Motif]. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart. | MRGPRX2 has an <scene name='90/904306/Erc_motif_3/1'>ERC Motif</scene> rather than the typically [https://proteopedia.org/wiki/index.php/A_Physical_Model_of_the_%CE%B22-Adrenergic_Receptor#conserved%20DRY%20motif conserved E/DRY Motif]. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart. | ||
[[Image:Screen Shot 2022-03-15 at 10.23.20 AM.png|200px|left|thumb|ERC Motif]] | [[Image:Screen Shot 2022-03-15 at 10.23.20 AM.png|200px|left|thumb|ERC Motif]] | ||
| - | |||
| - | |||
| Line 86: | Line 87: | ||
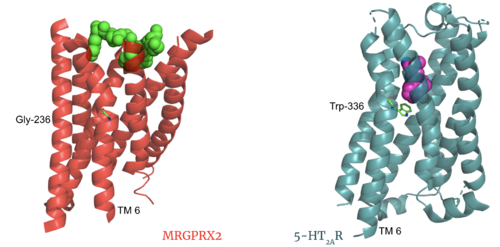
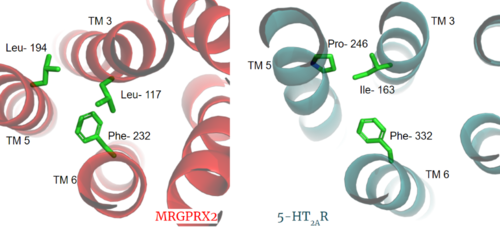
The MRGPRX2 disulfide bond is between <scene name='90/904305/Disulfide_bond/1'>Cys-168 and Cys-180</scene> on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the <scene name='90/904306/5ht2a_disulfide/2'>serotonin GPCR</scene> shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands. | The MRGPRX2 disulfide bond is between <scene name='90/904305/Disulfide_bond/1'>Cys-168 and Cys-180</scene> on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the <scene name='90/904306/5ht2a_disulfide/2'>serotonin GPCR</scene> shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands. | ||
[[Image:Screen Shot 2022-03-27 at 5.45.52 PM.png|300px|center|thumb|Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location.]] | [[Image:Screen Shot 2022-03-27 at 5.45.52 PM.png|300px|center|thumb|Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location.]] | ||
| - | |||
| - | |||
| - | |||
Revision as of 02:58, 29 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
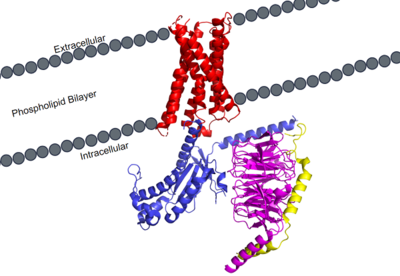
MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR)
| |||||||||||
References
- ↑ Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub, 2017 Oct 27. PMID:29075003 doi:http://dx.doi.org/10.1038/nrd.2017.178
- ↑ Basith S, Cui M, Macalino SJY, Park J, Clavio NAB, Kang S, Choi S. Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design. Front Pharmacol. 2018 Mar 9;9:128. doi: 10.3389/fphar.2018.00128. eCollection, 2018. PMID:29593527 doi:http://dx.doi.org/10.3389/fphar.2018.00128
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, Slocum ST, Krumm BE, Lansu K, McCorvy JD, Kroeze WK, English JG, DiBerto JF, Olsen RHJ, Huang XP, Zhang S, Liu Y, Kim K, Karpiak J, Jan LY, Abraham SN, Jin J, Shoichet BK, Fay JF, Roth BL. Structure, function and pharmacology of human itch GPCRs. Nature. 2021 Dec;600(7887):170-175. doi: 10.1038/s41586-021-04126-6. Epub 2021, Nov 17. PMID:34789874 doi:http://dx.doi.org/10.1038/s41586-021-04126-6
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021 Dec;600(7887):164-169. doi: 10.1038/s41586-021-04077-y. Epub 2021, Nov 17. PMID:34789875 doi:http://dx.doi.org/10.1038/s41586-021-04077-y
- ↑ Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, Brimble M, Osman N, Little PJ. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2015 Mar 24;2:14. doi: 10.3389/fcvm.2015.00014. eCollection, 2015. PMID:26664886 doi:http://dx.doi.org/10.3389/fcvm.2015.00014
- ↑ Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs--theoretical and experimental studies. Curr Med Chem. 2012;19(8):1090-109. doi: 10.2174/092986712799320556. PMID:22300046 doi:http://dx.doi.org/10.2174/092986712799320556
- ↑ Olivella M, Caltabiano G, Cordomi A. The role of Cysteine 6.47 in class A GPCRs. BMC Struct Biol. 2013 Mar 15;13:3. doi: 10.1186/1472-6807-13-3. PMID:23497259 doi:http://dx.doi.org/10.1186/1472-6807-13-3