We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1710
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
=== Closed Conformation === | === Closed Conformation === | ||
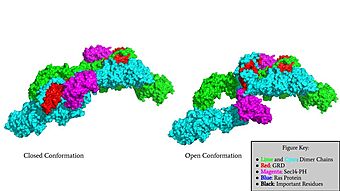
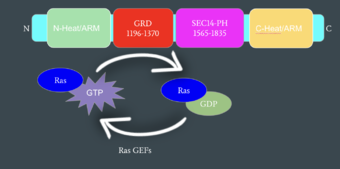
| - | The first conformation of Neurofibromin is known as the <scene name='90/904315/Closed/2'>Closed Conformation</scene>, which is representative of an inactive Neurofibromin protein. In the closed conformation, both sets of the GRD and Sec14-PH domains are rotated in a way that they are inaccessible and inactive due to a <scene name='90/904315/Catalytic_triade/2'>triade</scene> consisting of residues Cysteine 1032, Histidine 1558, and Histidine 1576 that form a transition metal-binding site with zinc. The close proximity of the C1032, H1558, and H1576 residues that form the <scene name='90/904316/Closed_triade/ | + | The first conformation of Neurofibromin is known as the <scene name='90/904315/Closed/2'>Closed Conformation</scene>, which is representative of an inactive Neurofibromin protein. In the closed conformation, both sets of the GRD and Sec14-PH domains are rotated in a way that they are inaccessible and inactive due to a <scene name='90/904315/Catalytic_triade/2'>triade</scene> consisting of residues Cysteine 1032, Histidine 1558, and Histidine 1576 that form a transition metal-binding site with zinc. The close proximity of the C1032, H1558, and H1576 residues that form the <scene name='90/904316/Closed_triade/1'>triade in the closed conformation</scene> keep the GRD domain packed tightly on top of the Neurofibromin core. This tight compaction leads to steric inhibition when Neurofibromin tries to perform its function and associate with Ras. The association between Ras and Neurofibromin is supposed to occur via an <scene name='90/904316/Arg_finger/1'>Arginine Finger</scene> located at Residue 1276. However, the steric hindrance from the Neurofibromin core in the closed conformation inhibits this association. Therefore, when Neurofibromin is in the closed conformation, there is no association with Ras and cell growth and proliferation is able to occur. |
=== Open Conformation === | === Open Conformation === | ||
| - | The other conformation that characterizes Neurofibromin is the <scene name='90/904315/Open_conformation/3'>Open Conformation</scene>. In the open conformation of Neurofibromin, the protein is considered active and is participating in its function of Ras regulation. This occurs because the transition metal-binding site with zinc no longer is able to form due to an increase in distance between the C1032, H1558 and H1576 residues that form the <scene name='90/904315/Open_conformation_triade/ | + | The other conformation that characterizes Neurofibromin is the <scene name='90/904315/Open_conformation/3'>Open Conformation</scene>. In the open conformation of Neurofibromin, the protein is considered active and is participating in its function of Ras regulation. This occurs because the transition metal-binding site with zinc no longer is able to form due to an increase in distance between the C1032, H1558 and H1576 residues that form the <scene name='90/904315/Open_conformation_triade/4'>Open Triade</scene>. One protomer in Neurofibromin has its GRD and Sec14-PH domains oriented in way that is almost reversed in position compared to the closed conformation. Due to this rotation, C1032 is now located too far away, approximately 30 Angstroms, from H1558 and H1576 which results in the loss of the metal-binding site. The lack of the transition metal-binding site allows the GRD to orient itself in such a way that it can associate with <scene name='90/904315/Ras_open_conformation/1'>Ras in the Open Conformation</scene>. The reason that Neurofibromin is only able to associate with Ras in the open conformation is due to one critical residue, the <scene name='90/904315/Open_conformation_arginine_fin/1'>Arginine Finger</scene> located at position 1276 in Neurofibromin. When Neurofibromin is in the open conformation, R1276 is able to <scene name='90/904315/Ras_open_conformation_with_arg/1'>bind to Ras</scene> because there is no steric hindrance from the Neurofibromin core. |
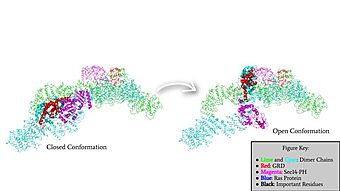
[[Image:GRDandSec14PHRotation.jpg|340 px|left|thumb|Rotation of the GRD and Sec14-PH domains from the closed conformation of neurofibromin to the open conformation of neurofibromin to allow Ras binding. The GRD rotates -130° and the Sec14-PH domain rotates -90°]] | [[Image:GRDandSec14PHRotation.jpg|340 px|left|thumb|Rotation of the GRD and Sec14-PH domains from the closed conformation of neurofibromin to the open conformation of neurofibromin to allow Ras binding. The GRD rotates -130° and the Sec14-PH domain rotates -90°]] | ||
Revision as of 18:52, 29 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Human Neurofibromin - The Tumor Suppressor Gene
| |||||||||||
References
- ↑ Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006 Jul;70(1):1-13. doi: 10.1111/j.1399-0004.2006.00639.x. PMID:16813595 doi:http://dx.doi.org/10.1111/j.1399-0004.2006.00639.x
- ↑ Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ 4.0 4.1 4.2 Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015 May;15(5):290-301. doi: 10.1038/nrc3911. Epub 2015 Apr 16. PMID:25877329 doi:http://dx.doi.org/10.1038/nrc3911
- ↑ Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014 Jul-Sep;18(3):297-306. PMID:25182393