We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1724
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
=== Vitamin K Cycle === | === Vitamin K Cycle === | ||
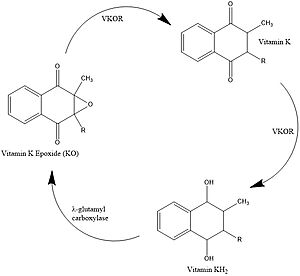
| - | Vitamin K is essential for blood clotting in the body. The fully reduced form, KH2, allows the gamma carboxylation of blood clotting cofactors and is turned into the epoxide form in the process. Vitamin K epoxide reductase turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps including converting the epoxide to partially oxidized Vitamin K then converting the partially oxidized Vitamin K to the fully reduced hydroquinone. <ref name="Stafford">PMID:16102054</ref> | + | [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] is essential for blood clotting in the body. The fully reduced form, KH2, allows the gamma carboxylation of blood clotting cofactors and is turned into the epoxide form in the process. Vitamin K epoxide reductase turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps including converting the epoxide to partially oxidized Vitamin K then converting the partially oxidized Vitamin K to the fully reduced hydroquinone. <ref name="Stafford">PMID:16102054</ref> |
=== Structural Overview === | === Structural Overview === | ||
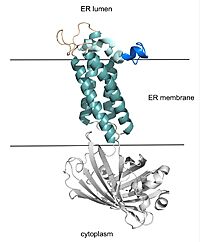
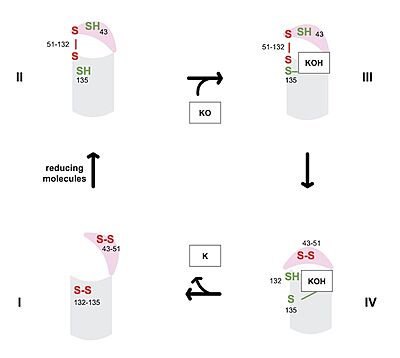
VKOR consists of four <scene name='90/904330/Transmembrane_helices/1'>transmembrane helices</scene> embedded in the endoplasmic reticulum membrane. The files on the RCSB Protein Data Bank include a barrel domain that is not pertinent to the function of VKOR. The images presented here have been edited to remove the barrel domain and renumbered to correspond with the article by Liu. <ref name="Liu">PMID:33154105</ref>. The original image with the barrel domain in context is shown in Figure 2. Helices one and two are connected by the beta hairpin region which contains two of the active cysteines, C43 and C51. VKOR also has a cap domain covering the active site, made up of an anchor, loop, and helix. | VKOR consists of four <scene name='90/904330/Transmembrane_helices/1'>transmembrane helices</scene> embedded in the endoplasmic reticulum membrane. The files on the RCSB Protein Data Bank include a barrel domain that is not pertinent to the function of VKOR. The images presented here have been edited to remove the barrel domain and renumbered to correspond with the article by Liu. <ref name="Liu">PMID:33154105</ref>. The original image with the barrel domain in context is shown in Figure 2. Helices one and two are connected by the beta hairpin region which contains two of the active cysteines, C43 and C51. VKOR also has a cap domain covering the active site, made up of an anchor, loop, and helix. | ||
Revision as of 22:52, 29 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ 2.0 2.1 Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
Student Contributors
Izabella Jordan, Emma Varness