This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Methionine synthase
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
This page is being worked on during the Spring 2022 semester. | This page is being worked on during the Spring 2022 semester. | ||
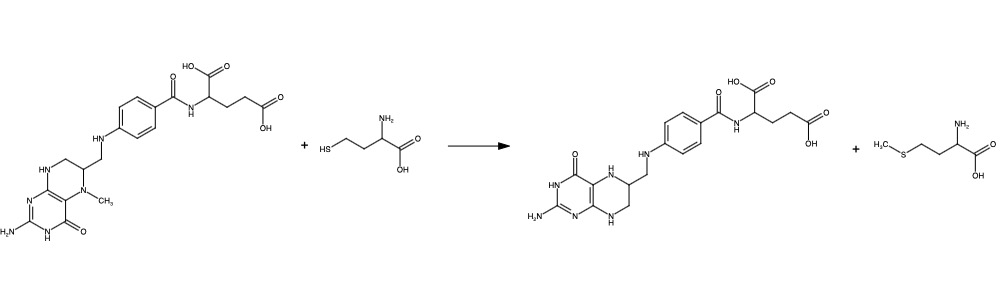
| - | Methionine is an essential amino acid required in order for our bodies to have healthy cell and tissue growth. Unfortunately, it is not naturally derived and | + | Methionine is an essential amino acid required in order for our bodies to have healthy cell and tissue growth. Unfortunately, it is not naturally derived and dependent on our diets. Methionine synthase methylates homocysteine, another amino acid obtained typically by any meat we consume, to methionine<ref>DOI: 10.1038/nature10916</ref>. |
| + | |||
| + | The change from homocysteine to methionine is one methyl group, by which is received from methyltetrahydrofolate (MTHF), a product of Methylenetetrahydrofolate reductase (MTHFR), as the methyl donor and a protein-bound B-12 vitamin Cobalamin as the methyl carrier. | ||
EC: 2.1.1.13 | EC: 2.1.1.13 | ||
| + | |||
PDB ID: 1K7Y | PDB ID: 1K7Y | ||
Revision as of 15:17, 30 March 2022
Contents |
Methionine synthase
| |||||||||||
Vitamin B-12
Oxidation States of Cobalamin
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy