User:George G. Papadeas/Sandbox VKOR
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Introduction== | == Introduction== | ||
=== Biological Role === | === Biological Role === | ||
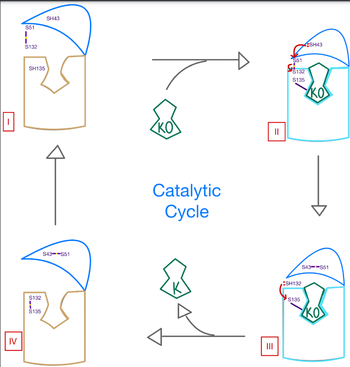
| - | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is an enzyme that reduces <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to | + | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is an enzyme that reduces <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] and ultimately Vitamin K hydroquinone (KH2) (cite fig 1). VKOR is a [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2919313/ 4-helix transmembrane protein] spanning the endoplasmic reticulum . One of its primary roles is to assist in blood coagulation through KH2 regeneration. KH2 is a necessary cofactor for the [https://www.britannica.com/science/bleeding/The-extrinsic-pathway-of-blood-coagulation#ref64617 γ-carboxylase] enzyme that activates several coagulation factors. |
Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like [https://pubmed.ncbi.nlm.nih.gov/33154105/ homologs]. | Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like [https://pubmed.ncbi.nlm.nih.gov/33154105/ homologs]. | ||
Revision as of 14:54, 5 April 2022
VKOR
| |||||||||||
References
1. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
2. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644