This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Methionine synthase
From Proteopedia
| Line 13: | Line 13: | ||
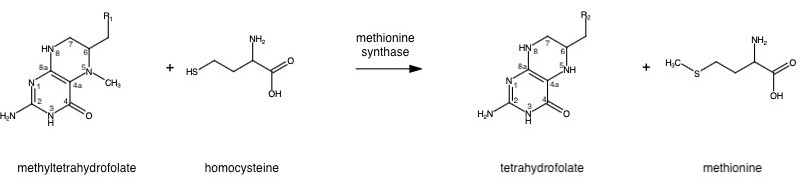
Methionine synthase (MetH) is a B12-dependent enzyme that methylates homocysteine to regenerate methionine. The change from homocysteine to methionine is a methyl group. This reaction is regulated by methyltetrahydrofolate (a product from MTFHR) as a methyl donor and B12 as the methyl carrier. | Methionine synthase (MetH) is a B12-dependent enzyme that methylates homocysteine to regenerate methionine. The change from homocysteine to methionine is a methyl group. This reaction is regulated by methyltetrahydrofolate (a product from MTFHR) as a methyl donor and B12 as the methyl carrier. | ||
| - | The | + | The <scene name='90/907471/Superposition_1/2'>full structure of MetH</scene> has yet to be determined but we understand it contains 4 domains for its catalytic and its reactivation cycles requiring B12 cobalamin (in pink), methyltetrahydrofolate (blue), homocysteine (yellow), and SAH (as part of the SAM cycle; in red). |
With activation of methionine synthase, the first domain of B12 is initially in a “capped” position preventing unwanted chemistry from occurring. To uncap the B12 domain, methyltransferase comes in to also assist with the methyl transfer from methyltetrahydrofolate allowing B12 to accept and carry the methyl. | With activation of methionine synthase, the first domain of B12 is initially in a “capped” position preventing unwanted chemistry from occurring. To uncap the B12 domain, methyltransferase comes in to also assist with the methyl transfer from methyltetrahydrofolate allowing B12 to accept and carry the methyl. | ||
Revision as of 18:30, 5 April 2022
Contents |
Methionine synthase
| |||||||||||
The change from homocysteine to methionine is an SN2 reaction where the methyl group from methyltetrahydrofolate (MTHF), located on N-5, is donated. MTHF is a product of MTHFR.
This is a complex reaction as the product, tetrahydrofolate, is a poor leaving group, thus requiring a "supernucleophile" with a protein-bound B-12 vitamin Cobalamin as the methyl carrier.
Vitamin B-12
Oxidation States of Cobalamin
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
References
- ↑ Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006 Oct;188(20):7195-204. doi: 10.1128/JB.00208-06. PMID:17015658 doi:http://dx.doi.org/10.1128/JB.00208-06
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy