This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Methionine synthase
From Proteopedia
| Line 1: | Line 1: | ||
==Methionine synthase== | ==Methionine synthase== | ||
| - | |||
<StructureSection load='1k7y' size='310' side='right' caption='B-12 dependent fragment of E. coli methionine synthase with Cobalt (in pink)' scene=''> | <StructureSection load='1k7y' size='310' side='right' caption='B-12 dependent fragment of E. coli methionine synthase with Cobalt (in pink)' scene=''> | ||
| Line 9: | Line 8: | ||
PDB ID: 1K7Y - cobalamin, 1K98 - AdoMet complex | PDB ID: 1K7Y - cobalamin, 1K98 - AdoMet complex | ||
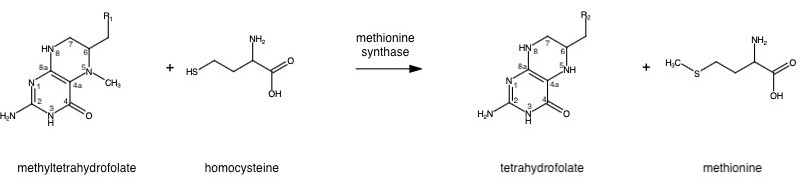
| - | Methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is essential as it is not naturally derived, and we get it from our diet in the form of homocysteine. | + | Methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is essential as it is not naturally derived, and we get it from our diet first in the form of homocysteine. |
| - | + | [[Image:Overall.jpeg]] | |
| - | The | + | The change from homocysteine to methionine is an SN2 reaction where the methyl group from methyltetrahydrofolate (MTHF), located on N-5, is donated. MTHF is a product of MTHFR. |
| + | |||
| + | This is a complex reaction as the product, tetrahydrofolate, is a poor leaving group, thus requiring a "supernucleophile" with a protein-bound B-12 vitamin Cobalamin as the methyl carrier. | ||
</StructureSection> | </StructureSection> | ||
| - | [[Image:Overall.jpeg]] | ||
| - | + | == Structural highlights == | |
| + | |||
| + | Methionine synthase (MetH) is a B12-dependent enzyme that methylates homocysteine to regenerate methionine. The <scene name='90/907471/Superposition_1/2'>full structure of MetH</scene> has yet to be determined but we understand it contains 4 domains of B12 cobalamin (in pink), methyltetrahydrofolate (blue), homocysteine (yellow), and SAH (as part of the SAM cycle; in red). Each domain with an important function required for catalytic and reactivation cycles. | ||
| + | |||
| - | This is a complex reaction as the product, tetrahydrofolate, is a poor leaving group, thus requiring a "supernucleophile" with a protein-bound B-12 vitamin Cobalamin as the methyl carrier. | ||
== Vitamin B-12 == | == Vitamin B-12 == | ||
| Line 29: | Line 31: | ||
== Relevance == | == Relevance == | ||
| - | + | Methionine deficiency can result in diseases such as birth abnormalities. | |
| + | |||
| + | |||
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Revision as of 18:35, 5 April 2022
Contents |
Methionine synthase
| |||||||||||
Structural highlights
Methionine synthase (MetH) is a B12-dependent enzyme that methylates homocysteine to regenerate methionine. The has yet to be determined but we understand it contains 4 domains of B12 cobalamin (in pink), methyltetrahydrofolate (blue), homocysteine (yellow), and SAH (as part of the SAM cycle; in red). Each domain with an important function required for catalytic and reactivation cycles.
Vitamin B-12
Oxidation States of Cobalamin
Relevance
Methionine deficiency can result in diseases such as birth abnormalities.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
References
- ↑ Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006 Oct;188(20):7195-204. doi: 10.1128/JB.00208-06. PMID:17015658 doi:http://dx.doi.org/10.1128/JB.00208-06
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy