Methionine synthase
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
== Structural highlights == | == Structural highlights == | ||
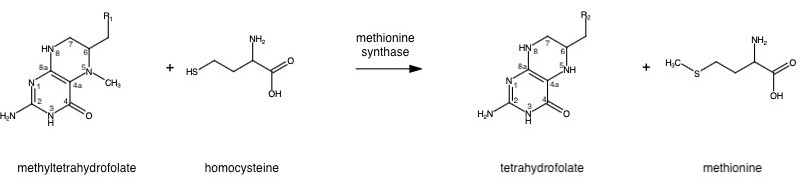
| - | Methionine synthase (MetH) is a B12-dependent enzyme that methylates homocysteine to regenerate methionine. The <scene name='90/907471/Superposition_1/2'>full structure of MetH</scene> has yet to be determined but we understand it contains 4 domains of B12 cobalamin (in pink), methyltetrahydrofolate (blue), homocysteine (yellow), and SAH (as part of the SAM cycle; in red). Each domain with an important function required for catalytic and reactivation cycles. | + | Methionine synthase (MetH) is a B12-dependent enzyme that methylates homocysteine to regenerate methionine. The <scene name='90/907471/Superposition_1/2'>full structure of MetH</scene> has yet to be determined but we understand it contains 4 domains of B12 cobalamin (in pink), methyltetrahydrofolate (blue), homocysteine (yellow), and SAH (as part of the SAM cycle; in red). Each domain with an important function required for catalytic and reactivation cycles<ref>DOI: 10.1038/nsb738</ref>. |
</StructureSection> | </StructureSection> | ||
== Vitamin B-12 == | == Vitamin B-12 == | ||
| - | |||
| Line 34: | Line 33: | ||
== Relevance == | == Relevance == | ||
| - | Methionine deficiency can result in diseases such as birth abnormalities. | + | Methionine deficiency can result in diseases such as birth abnormalities<ref>DOI: 10.1038/nature10916</ref>. |
== References == | == References == | ||
<ref>DOI: 10.1128/JB.00208-06</ref> | <ref>DOI: 10.1128/JB.00208-06</ref> | ||
| + | |||
<references/> | <references/> | ||
Revision as of 18:40, 5 April 2022
Contents |
Methionine synthase
| |||||||||||
Vitamin B-12
Oxidation States of Cobalamin
Co(I) - active, unstable, high energy
Co(II) - common oxidation state
Relevance
Methionine deficiency can result in diseases such as birth abnormalities[2].
References
- ↑ Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
- ↑ Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006 Oct;188(20):7195-204. doi: 10.1128/JB.00208-06. PMID:17015658 doi:http://dx.doi.org/10.1128/JB.00208-06
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy