We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1710
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
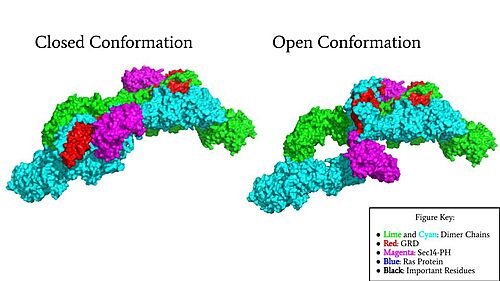
[[Image:Neurofibromin Surface w Labels.jpg|500 px|right|thumb|Figure 1: Surface Rendering of Neurofibromin in its Open (7PGT) and Closed (7PGR) Conformation.]] | [[Image:Neurofibromin Surface w Labels.jpg|500 px|right|thumb|Figure 1: Surface Rendering of Neurofibromin in its Open (7PGT) and Closed (7PGR) Conformation.]] | ||
Neurofibromin is encoded by [https://en.wikipedia.org/wiki/Neurofibromin_1 NF1 gene], located on chromosome 17. Neurofibromin functions as a tumor suppressor through its association with the protein [https://proteopedia.org/wiki/index.php/Ras Ras]. The molecular structure of Neurofibromin has been determined by [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo-Electron Microscopy]. The structure of neurofibromin isoform 2 revealed different functional states for the Neurofibromin protein.<ref name="Naschberger">PMID:34707296</ref> Mutations in Neurofibromin are associated with diseases such as [https://en.wikipedia.org/wiki/Neurofibroma Plexiform Neurofibromas]. (FLESH OUT INTRODUCTION) | Neurofibromin is encoded by [https://en.wikipedia.org/wiki/Neurofibromin_1 NF1 gene], located on chromosome 17. Neurofibromin functions as a tumor suppressor through its association with the protein [https://proteopedia.org/wiki/index.php/Ras Ras]. The molecular structure of Neurofibromin has been determined by [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo-Electron Microscopy]. The structure of neurofibromin isoform 2 revealed different functional states for the Neurofibromin protein.<ref name="Naschberger">PMID:34707296</ref> Mutations in Neurofibromin are associated with diseases such as [https://en.wikipedia.org/wiki/Neurofibroma Plexiform Neurofibromas]. (FLESH OUT INTRODUCTION) | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
== Function == | == Function == | ||
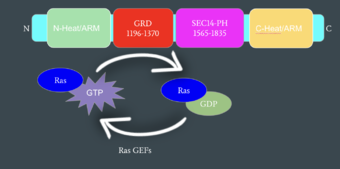
| + | [[Image:Neurofibromin_Cartoon_Domains.jpg|500 px|left|thumb|Figure 2: Neurofibromin Important Domains]] | ||
Neurofibromin functions as a tumor suppressor protein.<ref name="Trovó-Marqui">PMID:16813595</ref> It prevents cell growth by turning off [https://en.wikipedia.org/wiki/Ras_GTPase Ras] which in its active state, stimulates cell growth and division. Ras is a small, monomeric [https://en.wikipedia.org/wiki/GTPase GTPase]. Ras is membrane-bound and interacts with Neurofibromin, a cytoplasmic protein, in the open conformation of Neurofibromin. (DEFINE RAS) Neurofibromin is brought to the membrane to associate with Ras by [https://medlineplus.gov/genetics/gene/spred1/ SPRED1]. (DEFINE SPRED1) Unlike Ras, Neurofibromin can interact with SPRED1 in both the open and closed conformations. The interaction between Neurofibromin and Ras is activated via an [https://en.wikipedia.org/wiki/Arginine_finger Arginine finger] (Arg 1276) present in the GRD domain of Neurofibromin. Arg 1276 is only accessible for binding when the GRD and Sec14-PH domains are rotated into the open conformation. When Arg 1276 is able to associate with Ras, Neurofibromin downregulates the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway Ras signaling pathway] by speeding up Ras's GTPase activity, hydrolyzing the GTP associated with Ras to GDP. In its GDP bound state, Ras is inactive and cell growth and division are inhibited. (MECHANISTIC EXPLANATION). | Neurofibromin functions as a tumor suppressor protein.<ref name="Trovó-Marqui">PMID:16813595</ref> It prevents cell growth by turning off [https://en.wikipedia.org/wiki/Ras_GTPase Ras] which in its active state, stimulates cell growth and division. Ras is a small, monomeric [https://en.wikipedia.org/wiki/GTPase GTPase]. Ras is membrane-bound and interacts with Neurofibromin, a cytoplasmic protein, in the open conformation of Neurofibromin. (DEFINE RAS) Neurofibromin is brought to the membrane to associate with Ras by [https://medlineplus.gov/genetics/gene/spred1/ SPRED1]. (DEFINE SPRED1) Unlike Ras, Neurofibromin can interact with SPRED1 in both the open and closed conformations. The interaction between Neurofibromin and Ras is activated via an [https://en.wikipedia.org/wiki/Arginine_finger Arginine finger] (Arg 1276) present in the GRD domain of Neurofibromin. Arg 1276 is only accessible for binding when the GRD and Sec14-PH domains are rotated into the open conformation. When Arg 1276 is able to associate with Ras, Neurofibromin downregulates the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway Ras signaling pathway] by speeding up Ras's GTPase activity, hydrolyzing the GTP associated with Ras to GDP. In its GDP bound state, Ras is inactive and cell growth and division are inhibited. (MECHANISTIC EXPLANATION). | ||
[[Image:mechanismofRas.png|340 px|right|thumb|Mechanism of Ras Regulation by Neurofibromin]] | [[Image:mechanismofRas.png|340 px|right|thumb|Mechanism of Ras Regulation by Neurofibromin]] | ||
== Structure == | == Structure == | ||
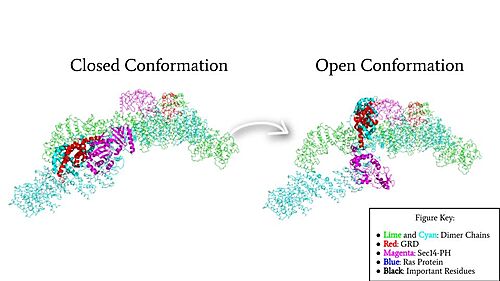
| - | [[Image:Neurofibromin_Cartoon_Domains.jpg|500 px|thumb|Figure 2: Neurofibromin Important Domains]] | ||
Neurofibromin is a <scene name='90/904315/Homodimer/5'>homodimer</scene> made up of two identical chains. Neurofibromin has two conformations, open and closed. Shifting between these controls neurofibromin's ability to associate with Ras and perform its function of Ras regulation. The transformation between the overall closed and open conformations transitions it from an active to inactive state. There are <scene name='90/904315/Sec14ph_and_grd_domain/1'>two important domains</scene> involved in the transition between the open and closed conformations, the <scene name='90/904316/Grd_domains/2'>GRD</scene> domain and the <scene name='90/904315/Sec14ph_domain/3'>Sec14-PH</scene> domain. Although neurofibromin is a homodimer with two identical protomers, only one protomer has its GRD and Sec14-PH domains rotated into the open conformation. | Neurofibromin is a <scene name='90/904315/Homodimer/5'>homodimer</scene> made up of two identical chains. Neurofibromin has two conformations, open and closed. Shifting between these controls neurofibromin's ability to associate with Ras and perform its function of Ras regulation. The transformation between the overall closed and open conformations transitions it from an active to inactive state. There are <scene name='90/904315/Sec14ph_and_grd_domain/1'>two important domains</scene> involved in the transition between the open and closed conformations, the <scene name='90/904316/Grd_domains/2'>GRD</scene> domain and the <scene name='90/904315/Sec14ph_domain/3'>Sec14-PH</scene> domain. Although neurofibromin is a homodimer with two identical protomers, only one protomer has its GRD and Sec14-PH domains rotated into the open conformation. | ||
Revision as of 14:39, 12 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Human Neurofibromin - The Tumor Suppressor Gene
| |||||||||||
References
- ↑ Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006 Jul;70(1):1-13. doi: 10.1111/j.1399-0004.2006.00639.x. PMID:16813595 doi:http://dx.doi.org/10.1111/j.1399-0004.2006.00639.x
- ↑ Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014 Jul-Sep;18(3):297-306. PMID:25182393
- ↑ Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015 May;15(5):290-301. doi: 10.1038/nrc3911. Epub 2015 Apr 16. PMID:25877329 doi:http://dx.doi.org/10.1038/nrc3911