User:George G. Papadeas/Sandbox VKOR
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Introduction== | == Introduction== | ||
=== Biological Role === | === Biological Role === | ||
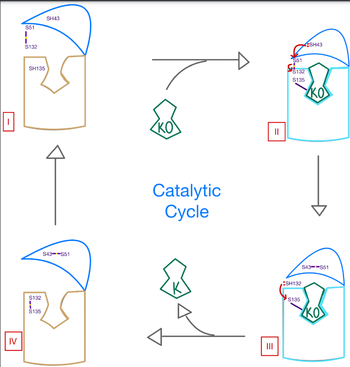
| - | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein <ref>DOI 10.1126</ref>. Its enzymatic role is reducing <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] | + | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein <ref>DOI 10.1126</ref>. Its enzymatic role is reducing <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K hydroquinone] (KH2) (Figure 1). The mechanism first occurs through the binding KO and using two cysteine residues to reduce KO into Vitamin K. Then, a second pair of cysteine residues will reduce Vitamin K into the final product, KH2 (Figure 1). One of VKORs primary roles is to assist in blood coagulation through this KH2 regeneration mechanism. With Vitamin K as a cofactor, the [https://www.britannica.com/science/bleeding/The-extrinsic-pathway-of-blood-coagulation#ref64617 γ-carboxylase] enzyme will enact post-translational modification on KH2, oxidizing it back to KO. The oxidation of KH2 by γ-carboxylase is coupled with the carboxylation of a glutamate residue to form γ-carboxyglutamate. The coupling of this oxidation and carboxylation will activate several clotting factors in the coagulation cascade. |
| + | Catalytic Mech Pic.png | ||
=== Author's Notes === | === Author's Notes === | ||
| Line 16: | Line 17: | ||
=== Active Site === | === Active Site === | ||
| - | Within the four transmembrane helices lies the <scene name='90/906893/Active_site/4'>binding pocket</scene><scene name='90/906893/Active_site/5'>active site</scene>. The active site is comprised of a hydrophobic pocket containing two hydrophilic residues, N80 and Y139, that interact with substrates and ligands alike. The hydrophobic pocket provides specificity to the region while the hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The C132-C135 disulfide bridge above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. Upon binding, VKOR will transition into the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> allowing the catalytic mechanism to commence. | + | Within the four transmembrane helices lies the <scene name='90/906893/Active_site/4'>binding pocket</scene><scene name='90/906893/Active_site/5'>active site</scene>. The active site is comprised of a hydrophobic pocket containing <scene name='90/906893/Active_site/7'>two hydrophilic residues</scene>, N80 and Y139, that interact with substrates and ligands alike. The hydrophobic pocket provides specificity to the region while the hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The C132-C135 disulfide bridge above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. Upon binding, VKOR will transition into the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> allowing the catalytic mechanism to commence. |
=== Cap Domain === | === Cap Domain === | ||
Revision as of 21:31, 13 April 2022
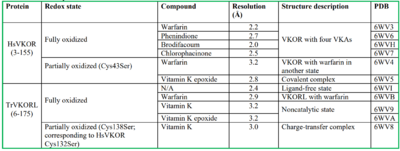
VKOR
| |||||||||||
References
1. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
2. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Unknown PubmedID 10.1126
- ↑ Unknown PubmedID 10.1126