Sandbox Reserved 1700
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
== GPCR Structure == | == GPCR Structure == | ||
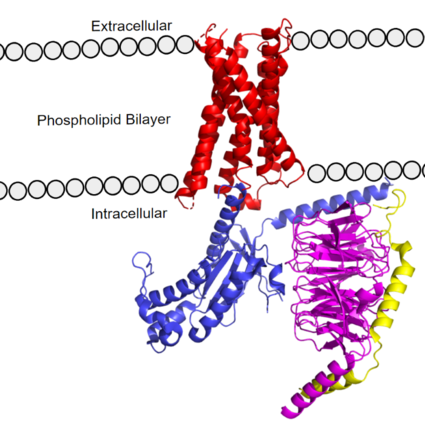
| - | The MRGPRX2 receptor structure was determined by cryo-electron microscopy (cryo-EM) <ref name= "Cao" /> <ref name= "Yang" />. Despite MRGPRX2’s novel characteristics, these structures still confirmed MRGPRX2 classification as an A-family GPCR. MRGPRX2 therefore shares the same general structural domains of all GPCR’s. This includes a <scene name='90/904305/Structure_overview_red/3'>transmembrane domain</scene> that interacts with a heterotrimeric <scene name='90/904305/Structure_overview_gprotein/2'>G-protein</scene> domain, consisting of <scene name='90/904305/Structure_overview_alpha/2'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/2'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/ | + | The MRGPRX2 receptor structure was determined by cryo-electron microscopy (cryo-EM) <ref name= "Cao" /> <ref name= "Yang" />. Despite MRGPRX2’s novel characteristics, these structures still confirmed MRGPRX2 classification as an A-family GPCR. MRGPRX2 therefore shares the same general structural domains of all GPCR’s. This includes a <scene name='90/904305/Structure_overview_red/3'>transmembrane domain</scene> that interacts with a heterotrimeric <scene name='90/904305/Structure_overview_gprotein/2'>G-protein</scene> domain, consisting of <scene name='90/904305/Structure_overview_alpha/2'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/2'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/2'>gamma</scene> subunits. The G-protein serves as the intracellular relay for ligand binding to the receptor. In preparing the protein sample, MRGPRX2 was prepared with an <scene name='90/904305/Antibody_representation/1'>antibody scFv16</scene> in order to stabilize the transmembrane domain for proper imaging. For simplicity and to focus on the MRGPRX2 receptor, the antibody has been removed in structural scenes. |
=== Transmembrane Domain === | === Transmembrane Domain === | ||
| Line 25: | Line 25: | ||
=== G-Protein === | === G-Protein === | ||
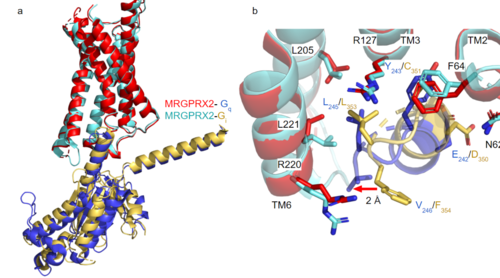
| - | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of <scene name=''90/904305/Structure_overview_alpha/2'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/2'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/ | + | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of <scene name=''90/904305/Structure_overview_alpha/2'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/2'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/2'>gamma</scene> subunits that interact with the intracellular transmembrane region at an <scene name='90/904306/Interface_2/1'>interface</scene> ( '''Figure 2b'''). G-proteins are responsible for transmitting extracellular signals into the cell upon activation. Activation leads to a substitution of GDP with GTP within the alpha subunit, causing the alpha subunit to disassociate from the beta and gamma subunits to initiate an intracellular signaling cascade. There are different families of G-alpha subunits, Gαi, Gαs, Gα12/13, and Gαq <ref name="Kamato">PMID: 26664886</ref>. MRGPRX2 binds to both Gαi and Gαq subunits with nearly identical structures despite slightly different amino acids present ( '''Figure 2a''') <ref name= "Cao" /> <ref name= "Yang" />. Throughout this page, MGPRX2 is always shown with Gq. The major difference between the Gq and Gi bound structures comes from one amino acid difference (valine on Gq versus phenylalanine on Gi) that pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. |
[[Image:Gq and gi overlay.png|500px|center|thumb|'''Figure 2a.''' Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow). '''Figure 2b.''' Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.]] | [[Image:Gq and gi overlay.png|500px|center|thumb|'''Figure 2a.''' Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow). '''Figure 2b.''' Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.]] | ||
Revision as of 20:05, 16 April 2022
MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR)
| |||||||||||
References
- ↑ Tuteja N. Signaling through G protein coupled receptors. Plant Signal Behav. 2009 Oct;4(10):942-7. doi: 10.4161/psb.4.10.9530. Epub 2009, Oct 14. PMID:19826234 doi:http://dx.doi.org/10.4161/psb.4.10.9530
- ↑ Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub, 2017 Oct 27. PMID:29075003 doi:http://dx.doi.org/10.1038/nrd.2017.178
- ↑ 3.0 3.1 Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol. 2018 Dec 20;9:3027. doi: 10.3389/fimmu.2018.03027. eCollection, 2018. PMID:30619367 doi:http://dx.doi.org/10.3389/fimmu.2018.03027
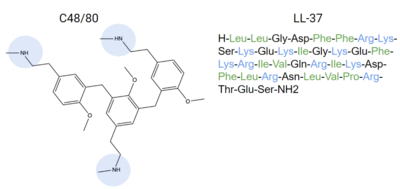
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Dondalska A, Ronnberg E, Ma H, Palsson SA, Magnusdottir E, Gao T, Adam L, Lerner EA, Nilsson G, Lagerstrom M, Spetz AL. Amelioration of Compound 48/80-Mediated Itch and LL-37-Induced Inflammation by a Single-Stranded Oligonucleotide. Front Immunol. 2020 Sep 30;11:559589. doi: 10.3389/fimmu.2020.559589. eCollection, 2020. PMID:33101278 doi:http://dx.doi.org/10.3389/fimmu.2020.559589
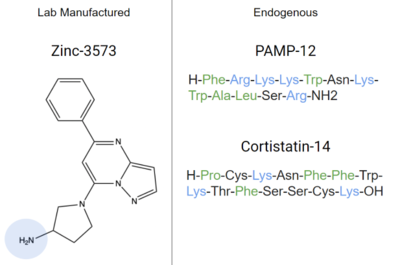
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015 Mar 12;519(7542):237-41. doi: 10.1038/nature14022. Epub 2014 Dec 17. PMID:25517090 doi:http://dx.doi.org/10.1038/nature14022
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, Slocum ST, Krumm BE, Lansu K, McCorvy JD, Kroeze WK, English JG, DiBerto JF, Olsen RHJ, Huang XP, Zhang S, Liu Y, Kim K, Karpiak J, Jan LY, Abraham SN, Jin J, Shoichet BK, Fay JF, Roth BL. Structure, function and pharmacology of human itch GPCRs. Nature. 2021 Dec;600(7887):170-175. doi: 10.1038/s41586-021-04126-6. Epub 2021, Nov 17. PMID:34789874 doi:http://dx.doi.org/10.1038/s41586-021-04126-6
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021 Dec;600(7887):164-169. doi: 10.1038/s41586-021-04077-y. Epub 2021, Nov 17. PMID:34789875 doi:http://dx.doi.org/10.1038/s41586-021-04077-y
- ↑ Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, Brimble M, Osman N, Little PJ. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2015 Mar 24;2:14. doi: 10.3389/fcvm.2015.00014. eCollection, 2015. PMID:26664886 doi:http://dx.doi.org/10.3389/fcvm.2015.00014
- ↑ Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs--theoretical and experimental studies. Curr Med Chem. 2012;19(8):1090-109. doi: 10.2174/092986712799320556. PMID:22300046 doi:http://dx.doi.org/10.2174/092986712799320556
- ↑ Olivella M, Caltabiano G, Cordomi A. The role of Cysteine 6.47 in class A GPCRs. BMC Struct Biol. 2013 Mar 15;13:3. doi: 10.1186/1472-6807-13-3. PMID:23497259 doi:http://dx.doi.org/10.1186/1472-6807-13-3
- ↑ Gonzalez-Rey E, Chorny A, Robledo G, Delgado M. Cortistatin, a new antiinflammatory peptide with therapeutic effect on lethal endotoxemia. J Exp Med. 2006 Mar 20;203(3):563-71. doi: 10.1084/jem.20052017. Epub 2006 Feb, 21. PMID:16492802 doi:http://dx.doi.org/10.1084/jem.20052017