MRGPRX2 is a certain type of GPCR that is located in the cellular membranes of mast cells.
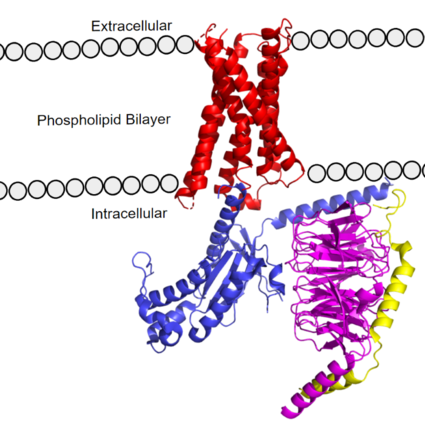
Figure 1. MRGPRX2 as it sits within the cellular membrane. Phospholipid bilayer is represented by grey dots, with labeled cellular locations
Background
GCPR’s or G-Protein Coupled Receptors are a large family of protein receptors that promote cellular signaling and signal transduction [1]. GPCRs transmit extracellular signals to intracellular messages. Many essential pathways utilize GPCRs, including human vision by the GPCR Rhodopsin, and the adrenaline fight-or-flight response by the β2-adrenoceptor GPCR. Understanding GPCR’s and how they produce their desired intracellular signal is essential to studying essential cellular pathways, especially in their diseased states. GCPRs are common drug targets, with 475 drugs acting on over 100 GPCRs. An additional 300 drugs are in clinical trial stages, and 20% of those drugs are targeting novel GPCRs [2]. Because of the clinical relevance of GPCRs, new structures provide new avenues for drug development to both treat disease or modulate the harmful side effects.
Some cells in the human body that express the MRGPRX2 receptor include mast cells in the skin, intestines, and trachea [3][4]. Mast cells are immune cells responsible for triggering inflammatory responses and are densely packed with granules containing inflammatory chemicals [4]. Mast cells can be activated by either antibodies from the immune response or upon ligands binding to MRGPRX2 receptors on their surface[5]. Upon activation, mast cells will release granules containing histamine and other inflammatory chemicals in the body, which can trigger a larger inflammatory response [4][5]. These responses induce common allergic reaction and anaphylaxis symptoms, such as cutaneous itching sensations or airway constriction
[6][7][5].
Ligands that bind to MRGPRX2 in the natural environment to produce an allergic response include exogenous molecules such as contents of insect venom, molecules like Compound 48/80 (C48/80) or other polycationic molecules[4] . They can also respond to endogenous signaling molecules involved in inflammation pathways such as cytokines, anaphylatoxins, or neuropeptides[3]. Many pseudo-allergic drug reactions have been tied to overactivity of MRGPRX2 receptors on mast cells[5], so research into receptor-ligand interactions of the MRGPRX2 receptor has the potential to mediate many adverse itching and allergic reaction side effects [5].
GPCRs are categorized into 6 different classes based on shared sequences and functions. MRGPRX2 is categorized into the Class A receptor family. However, itch receptors like MGPRX2 have unique structural features from most class A receptors [6][7]. These unique structural features, as seen in Class A Family Differences, cause conformational changes throughout the protein that impact what ligands bind to the receptor [7].
GPCR Structure
The MRGPRX2 receptor structure was determined by cryo-electron microscopy (cryo-EM) [6] [7]. Despite MRGPRX2’s novel characteristics, these structures still confirmed MRGPRX2 classification as an A-family GPCR. MRGPRX2 therefore shares the same general structural domains of all GPCR’s. This includes a that interacts with a heterotrimeric domain, consisting of , , and subunits. The G-protein serves as the intracellular relay for ligand binding to the receptor. In preparing the protein sample, MRGPRX2 was prepared with an in order to stabilize the transmembrane domain for proper imaging. For simplicity and to focus on the MRGPRX2 receptor, the antibody has been removed in structural scenes.
Transmembrane Domain
The transmembrane domain spans the cell membrane (Figure 1) and it consists of and (three extracellular loops, and three intracellular loops). The transmembrane helices are numbered 1-7 and contain special conserved motifs that are shared across other A family receptors. These motifs are expanded upon later, as they heavily contribute to the structure and therefore function of the transmembrane domain as a whole.
The extracellular region of the 7 transmembrane domain forms a single binding pocket with . Sub-pocket 1 is negatively charged due to negatively charged residues (Asp-184 and Glu-164), while sub-pocket 2 contains hydrophobic amino acids which contribute to hydrophobic interactions between the ligand and protein.
The intracellular region (Figure 1) is what connects the transmembrane helices with the G-protein.
G-Protein
GTP-binding proteins, also known as G-proteins, are heterotrimeric complexes consisting of , , and subunits that interact with the intracellular transmembrane region at an ( Figure 2b). G-proteins are responsible for transmitting extracellular signals into the cell upon activation. Activation leads to a substitution of GDP with GTP within the alpha subunit, causing the alpha subunit to disassociate from the beta and gamma subunits to initiate an intracellular signaling cascade. There are different families of G-alpha subunits, Gαi, Gαs, Gα12/13, and Gαq [8]. MRGPRX2 binds to both Gαi and Gαq subunits with nearly identical structures despite slightly different amino acids present ( Figure 2a) [6] [7]. Throughout this page, MGPRX2 is always shown with Gq. The major difference between the Gq and Gi bound structures comes from one amino acid difference (valine on Gq versus phenylalanine on Gi) that pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.
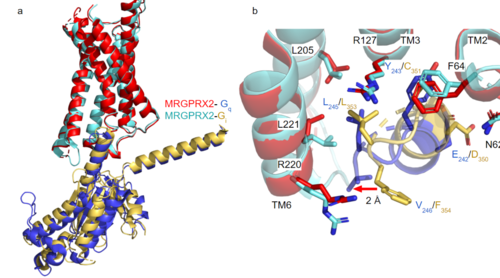
Figure 2a. Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow).
Figure 2b. Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.
Class A Family Differences
The most important findings about the MRGPRX2 receptor are the differences between it and all other previously discovered GPCR's found in the A family. Many conserved structural motifs, characteristic of the A family receptors, are absent on MRGPRX2. These structural motif differences contribute to a membrane surface ligand binding rather than a ligand binding deep within the helices (Figure 3). To demonstrate this difference in depth binding, MRGPRX2 is compared to 5-HT2AR, another class A GPCR with more conserved structural motifs.
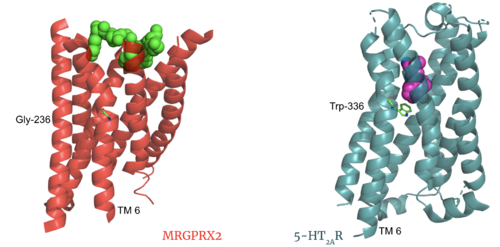
Figure 3. Comparison of ligand Cortistatin-14 binding in MRGPRX2 (left) and binding in 5HT2AR (right)
Toggle Switch
Certain residues that lie in the ligand binding pocket can act as molecular switches to turn the GPCR “on” or “off” and are fittingly called toggle switches. Toggle switches in receptors are essential in interacting with the ligand upon binding, as they can then initiate the transmission of the molecular signal through the protein. Trp-336 has been known to act as this "iconic" toggle switch in GPCR’s of the A family [9], and is also an important residue in another motif, known as the CWxP motif. However in MRGPRX2, the residue has been replaced to a [6] [7]. This is a significant modification to the receptor structure. By replacing the large tryptophan residue with a small glycine, the membrane helicies, especially helix 7 on which the toggle switch is found, can pack closer together. The ligands that interact with MRGPRX2 then bind much of the receptor, as opposed to deeper within the helices. This significant change can be visualized in Figure 3. This significantly changes what structures of ligands are able to interact with this receptor and therefore what types of molecules can activate the Human Itch GPCR. More details about what kinds of ligands bind to this receptor are discussed later.
Sodium Site
The MRGPRX2 consists of ASP-75 and GLY-116 compared to the previously conserved residues in this binding pocket. Other class A GPCRs demonstrate a larger binding pocket with a higher negative character allowing for a suitable environment for sodium ions to bind. In MRGPRX2, this pocket lacks the same amount of with the shift to a glycine residue rather than typical negative residues. The helices for the MRGPRX2 in the binding pocket are also more collapsed making this pocket less accessible for sodium ions.
PIF/LLF Motif
Another motif found in most, but not all, A family GPCR’s is the PIF motif. The three residues are found on transmembrane helices 5, 3, and 6, respectively. In MRGPRX2, the PIF motif is changed to LLF residues. Figure 3 shows the conserved PIF motif on 5HT2AR, compared to the LLF motif on MRGPRX2, found at on transmembrane helices 5, 3, and 6, respectively. This contributes to shifting helix 6 towards helix 3, and contributes to the tighter packing of helices [6] [7] and therefore a more surface-level ligand binding site.
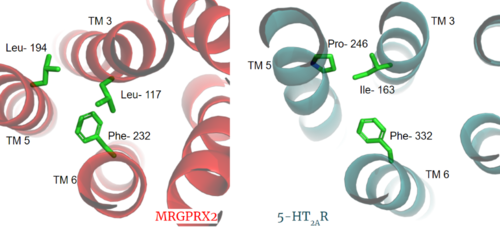
Figure 3. Conserved PIF motif in 5HT2AR (teal) compared to the LLF motif found in MRGPRX2 (red). Transmembrane helices and residues are numbered and labeled to show how this structural change shifts the orientation of the helices.
DRY/ ERC Motif
MRGPRX2 has an rather than the typically conserved E/DRY Motif. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart.
Disulfide Bonds
The MRGPRX2 disulfide bond is between on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands.

Figure 5. Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location.
Additional Structural Motifs
The MRGPRX2 GPCR also contains semi-conserved or fully-conserved motifs that are seen in other class A GPCRs.
NPxxY Motif
The residues in the are pivotal for receptor activation in all Class A GPCRs. This motif is conserved in the MRGPRX2 receptor with residues VAL-231, ASP-75, ASN-275, and TYR-279.
CWxP Motif
The is almost fully conserved except for TRP-236, or the toggle switch, which is replaced with GLY-236. CYS-235, LEU-237, and PRO-238 are all conserved. CYS6.47 may play a fundamental role in GPCR activity which would be supported by its conservation in MRGPRX2[10].
Ligands
MRGPRX2 binds a wide range of small molecule and peptide ligands. These ligands have positively charged regions which can interact with the negative binding pocket, sub-pocket 1. Some of these larger ligands also have a hydrophobic region which can interact with sub-pocket 2. MRGPRX2 is activated by C48/80[7][6] and LL-37 [4], among other endogenous inflammatory chemicals.
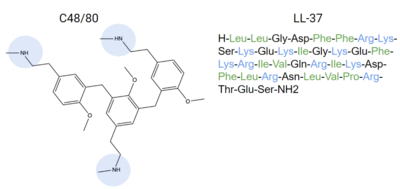
Figure 6. Endogenous MRGPRX2 ligands with positive regions in blue and hydrophobic regions in green. C48/80 and LL-37 are both released by the body as an allergic reaction response
[4]. These compounds can bind to MRGPRX2 and activate it, explaining how itching is a side effect of allergic reactions.
Other endogenous compounds that share similar characteristics of these ligands are Zinc-3573, PAMP-12, and Cortistatin-14. These molecules are known agonists of MRGPRX2 and bind similarly to sub-pockets 1 and 2.
An endogenous peptide, models these interactions, binding to sub-pocket 1 through LYS-3 and sub-pocket 2 through hydrophobic interactions.
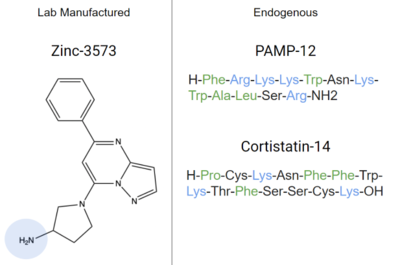
Figure 7. Agonists of MRGPRX2 due to their structural similarities to known ligands. Positive regions shown in blue and hydrophobic regions in green. Cortistatin-14, PAMP-12, and Zinc-3573 are all known to bind to MRGPRX2 with high affinity, and inhibit mast cell degranulation
[7][6]
Function
GPCRs undergo a transmembrane protein conformational change upon ligand binding. This signal is then transduced to the G-protein allowing for downstream responses due to between the alpha subunit of the G-protein and the transmembrane protein.
Before Activation
The culmination of different motifs observed in MRGPRX2 compared to other class A GPCRs leads to external membrane ligand binding. The MRGPRX2 GPCR undergoes a much smaller conformational change upon ligand binding compared to other Class A GPCRs due to surface level binding versus deep helix binding. This contrast is represented in the photo below to show the unbound and bound transmembrane proteins of MRGPRX2 and 5-HT2A.

Figure 8. Overlay of unbound (transparent) and bound (opaque) transmembrane proteins of both MRGRPX2 (left) and 5-HT2AR (right).
After Activation
After ligand binding and transmembrane protein activation, this signal is sent to the alpha subunit of the G-protein which undergoes its own chemical and conformational changes. The alpha subunit is initially bound with GDP which then is physically replaced by GTP leading to conformational changes. These changes can be seen in this video of a G-protein alpha subunit activation derived from common rats.
Clinical Relevance
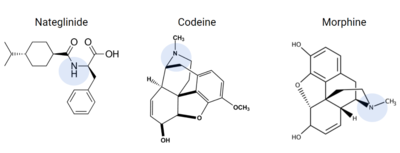
Figure 9. Common prescriptions that are tied to adverse itching side effects include nateglinide, morphine, and codeine. These drugs have structural similarities to known ligands for the MRGPRX2 receptor, and can lead to MRGPRX2 activation.
Many medications commonly list chronic skin itching or inflammatory responses as side effects, such as nateglinide, an anti-diabetic drug [6]. Other prescribed medications that list itching as a possible side effect are Atracurium, Rocuronium, Ciprofloxacin, and Levofloxacin[11], all of which are tied to MRGPRX2 activation.
Other drugs that have been known to trigger allergic reactions and even anaphylactic responses are opioids morphine or codeine[6], which could explain anaphylactic responses to anesthetics seen in some patients[5]. Upon analysis of these drugs, they share many structural similarities that are known to bind to the sub-pockets in MRGPRX2, shown in Figure 9, which is a possible explanation for the unwanted itch and inflammation responses produced in some patients when administering these drugs [5][6].
Possible treatments for these unwanted side effects of drugs can be developed by understanding the mechanism of the MRGPRX2 receptor. Research on Cortistatin-14, an endogenous neuropeptide, has shown that it has antiinflammatory properties because of how it binds to the MRGPRX2 receptor [12]. Additionally, Osthole, an extract from Cnidium monnieri plants, also known as Monnier's snowparsley, demonstrates similar MRGPRX2 inhibition because of its structural similarity to many known ligands for this receptor, and could possibly be used as a treatment option for adverse allergic reactions to commonly prescribed drugs (donstralta). More research on MRGPRX2 can open the door for the development of drugs that avoid activation of the MRGPRX2 receptor, or possible treatments for excessive inflammation and allergic reactions.

Figure 10.Schematic representation of MRGPRX2 triggering a cellular response and inflammation.
3D Structures
7s8l, MRGPRX2 Gq
7s8m, MRGPRX2 Gi
6wha, 5HT2AR











