Thymidylate synthase
From Proteopedia
| Line 14: | Line 14: | ||
Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH<sub>2</sub>H<sub>4</sub>F. Inhibition of TS halts the production of dTMP and, indirectly, 2'-deoxythymidine-5'-triphosphate (dTTP). Both dTMP and dTTP are essential building blocks for DNA synthesis and their absence halts the ability of cells to replicate their genetic information. This is especially effective in cancer cells that rapidly divide and require large amounts of dTMP and dTTP. <ref>DOI 10.2174/0929867054864868</ref> | Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH<sub>2</sub>H<sub>4</sub>F. Inhibition of TS halts the production of dTMP and, indirectly, 2'-deoxythymidine-5'-triphosphate (dTTP). Both dTMP and dTTP are essential building blocks for DNA synthesis and their absence halts the ability of cells to replicate their genetic information. This is especially effective in cancer cells that rapidly divide and require large amounts of dTMP and dTTP. <ref>DOI 10.2174/0929867054864868</ref> | ||
| - | == Structural highlights == | ||
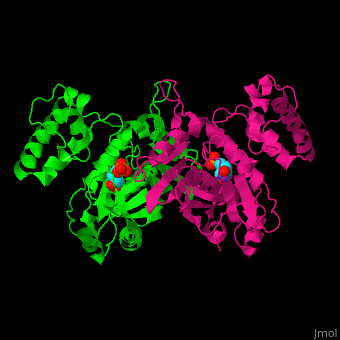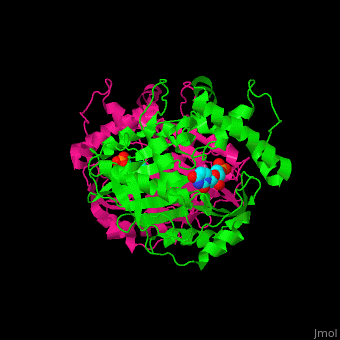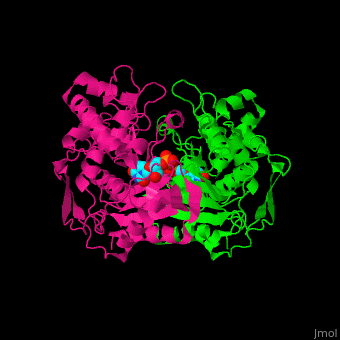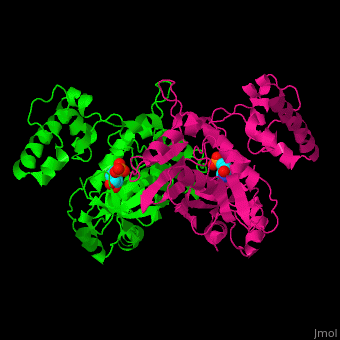
<StructureSection load='' size='350' side='right' caption='Thymidylate synthase complex with dUMP (PDB entry [[1tsv]])' scene='49/493689/Cv/1'> | <StructureSection load='' size='350' side='right' caption='Thymidylate synthase complex with dUMP (PDB entry [[1tsv]])' scene='49/493689/Cv/1'> | ||
| + | ==Structure and ligand binding== | ||
| + | Thymidylate synthase forms a dimer (reload <scene name='49/493689/Cv/1'>initial scene</scene>). | ||
TS <scene name='49/493689/Cv/4'>active site contains the substrate dUMP</scene><ref>PMID:9053905</ref>. Water molecules are shown as red spheres. | TS <scene name='49/493689/Cv/4'>active site contains the substrate dUMP</scene><ref>PMID:9053905</ref>. Water molecules are shown as red spheres. | ||
| Line 22: | Line 23: | ||
<jmolRadioGroup> | <jmolRadioGroup> | ||
<item> | <item> | ||
| - | <script>hide (VNM or 146:B) and altloc= | + | <script>hide (VNM or 146:B) and altloc=A</script> |
<text>Conformation 1</text> | <text>Conformation 1</text> | ||
| - | <checked> | + | <checked>true</checked> |
</item> | </item> | ||
<item> | <item> | ||
| - | <script>hide (VNM or 146:B) and altloc= | + | <script>hide (VNM or 146:B) and altloc=B</script> |
<text>Conformation 2</text> | <text>Conformation 2</text> | ||
<checked>false</checked> | <checked>false</checked> | ||
| Line 33: | Line 34: | ||
<item> | <item> | ||
<script>hide none</script> | <script>hide none</script> | ||
| - | <text>both | + | <text>both conformations</text> |
| - | <checked> | + | <checked>false</checked> |
</item> | </item> | ||
</jmolRadioGroup> | </jmolRadioGroup> | ||
</jmol> | </jmol> | ||
| + | ==Inhibition== | ||
| + | Methotrexate, a competitive inhibitor of folates, <scene name='49/493689/Inhibited/1'>inhibits</scene> TS. | ||
| + | </StructureSection> | ||
| + | |||
| + | |||
==3D structures of thymidylate synthase== | ==3D structures of thymidylate synthase== | ||
[[Thymidylate synthase 3D structures]] | [[Thymidylate synthase 3D structures]] | ||
| - | |||
| - | </StructureSection> | ||
== References == | == References == | ||
Revision as of 16:04, 18 April 2022
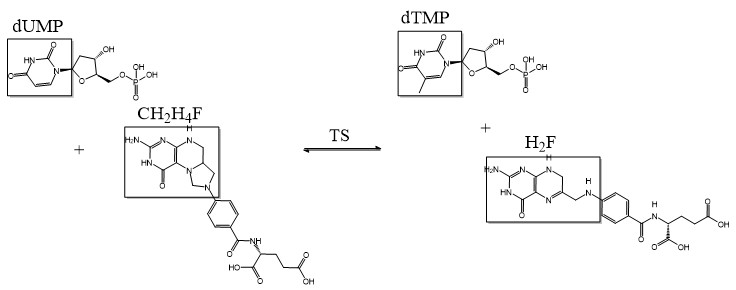
Thymidylate Synthase (TS) is an important enzyme in one-carbon metabolism. TS catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. [1]
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH2H4F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H2F)
Contents |
Function
Thymidylate synthase (TS) catalyzes the methylation of dUMP to dTMP using 5,10-methylenetetrahydrofolate as a cofactor. TS is essential for DNA replication and repair[2]. In protozoa, dihydrofolate reductase (DHFR) and TS are expressed as a bifunctional monomeric enzyme (DHFR-TS) with the DHFR entity at the N terminal. DHFR and TS catalyze consecutive reactions in the dTMP biosynthesis. There are two different types of TS – ThyA and ThyX. The types differ in their activity and structure. The TS ThyX are flavin-dependent enzymes.
Relevance
One of the nucleobase of DNA, cytosine, spontaneously deaminates to form uracil, changing the encoded message. Different from RNA, DNA does not contain uracil but instead contains the methylated form thymine, avoiding missense mutations. The DNA repair protein Uracil DNA glycosylase will recognize uracil as damaged DNA, removing it and re-establishing the original message[3]. Because thymine contains an additional methyl group, it is distinguishable from uracil, and is not removed through DNA repair. Thymidylate synthase helps to turn the abundant uridine (containing the nucleobase uracil) into deoxythymidine (containing the nucleobase thymine) in preparation for DNA synthesis prior to cell division. Thus, thymidylate synthase plays a role in making DNA a more reliable long-term storage of genetic information compared to RNA.
TS inhibition at its folate-binding site is used in anticancer therapeutic drugs. DHFR-TS inhibitors are potential drug targets against parasite-transferred diseases. TS exhibits oncogene-like activity.[4]
Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH2H4F. Inhibition of TS halts the production of dTMP and, indirectly, 2'-deoxythymidine-5'-triphosphate (dTTP). Both dTMP and dTTP are essential building blocks for DNA synthesis and their absence halts the ability of cells to replicate their genetic information. This is especially effective in cancer cells that rapidly divide and require large amounts of dTMP and dTTP. [5]
| |||||||||||
3D structures of thymidylate synthase
Thymidylate synthase 3D structures
References
- ↑ Kholodar SA, Finer-Moore JS, Swiderek K, Arafet K, Moliner V, Stroud RM, Kohen A. Caught in Action: X-ray Structure of Thymidylate Synthase with Noncovalent Intermediate Analog. Biochemistry. 2021 Apr 8. doi: 10.1021/acs.biochem.1c00063. PMID:33829766 doi:http://dx.doi.org/10.1021/acs.biochem.1c00063
- ↑ Kaneda S, Nalbantoglu J, Takeishi K, Shimizu K, Gotoh O, Seno T, Ayusawa D. Structural and functional analysis of the human thymidylate synthase gene. J Biol Chem. 1990 Nov 25;265(33):20277-84. PMID:2243092
- ↑ Krokan HE, Drablos F, Slupphaug G. Uracil in DNA--occurrence, consequences and repair. Oncogene. 2002 Dec 16;21(58):8935-48. doi: 10.1038/sj.onc.1205996. PMID:12483510 doi:http://dx.doi.org/10.1038/sj.onc.1205996
- ↑ Rahman L, Voeller D, Rahman M, Lipkowitz S, Allegra C, Barrett JC, Kaye FJ, Zajac-Kaye M. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004 Apr;5(4):341-51. PMID:15093541
- ↑ Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12(19):2241-58. doi: 10.2174/0929867054864868. PMID:16178783 doi:http://dx.doi.org/10.2174/0929867054864868
- ↑ Finer-Moore JS, Fauman EB, Morse RJ, Santi DV, Stroud RM. Contribution of a salt bridge to binding affinity and dUMP orientation to catalytic rate: mutation of a substrate-binding arginine in thymidylate synthase. Protein Eng. 1996 Jan;9(1):69-75. PMID:9053905
Proteopedia Page Contributors and Editors (what is this?)
Michael O'Shaughnessy, Karsten Theis, Michal Harel, Alexander Berchansky, Shaylie Albright, Anna Postnikova, Kia Yang, Joel L. Sussman