We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1700
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Background == | == Background == | ||
| - | [https://en.wikipedia.org/wiki/G_protein-coupled_receptor GCPR]’s or G-Protein Coupled Receptors are a large family of protein receptors that promote cellular signaling and signal transduction <ref name= “Tuteja”>PMID: 19826234</ref>. GPCRs transmit extracellular signals to intracellular messages. Many essential pathways utilize GPCRs, including human vision by the GPCR [https://proteopedia.org/wiki/index.php/Rhodopsin Rhodopsin], and the adrenaline fight-or-flight response by the [https://proteopedia.org/wiki/index.php/Beta2_adrenergic_receptor-Gs_protein_complex_updated β2-adrenoceptor GPCR]. Understanding GPCR’s and how they produce their desired intracellular signal is essential to studying essential cellular pathways, especially in their diseased states. GCPRs are common drug targets, with 475 drugs acting on over 100 GPCRs. An additional 300 drugs are in clinical trial stages, and 20% of those drugs are targeting novel GPCRs <ref name="Hauser">PMID:29075003</ref>. Because of the clinical relevance of GPCRs, new structures provide new avenues for drug development to both treat disease or modulate the harmful side effects. | + | [https://en.wikipedia.org/wiki/G_protein-coupled_receptor GCPR]’s or G-Protein Coupled Receptors are a large family of protein receptors that promote cellular signaling and [https://en.wikipedia.org/wiki/Signal_transduction signal transduction]<ref name= “Tuteja”>PMID: 19826234</ref>. GPCRs transmit extracellular signals to intracellular messages. Many essential pathways utilize GPCRs, including human vision by the GPCR [https://proteopedia.org/wiki/index.php/Rhodopsin Rhodopsin], and the adrenaline fight-or-flight response by the [https://proteopedia.org/wiki/index.php/Beta2_adrenergic_receptor-Gs_protein_complex_updated β2-adrenoceptor GPCR]. Understanding GPCR’s and how they produce their desired intracellular signal is essential to studying essential cellular pathways, especially in their diseased states. GCPRs are common drug targets, with 475 drugs acting on over 100 GPCRs. An additional 300 drugs are in clinical trial stages, and 20% of those drugs are targeting novel GPCRs <ref name="Hauser">PMID:29075003</ref>. Because of the clinical relevance of GPCRs, new structures provide new avenues for drug development to both treat disease or modulate the harmful side effects. |
| - | Some cells in the human body that express the MRGPRX2 receptor include [https://en.wikipedia.org/wiki/Mast_cell mast cells] in the skin, intestines, and trachea <ref name="Porebski">PMID:30619367</ref><ref name="Dondalska">PMID: 33101278</ref>. Mast cells are immune cells responsible for triggering inflammatory responses | + | Some cells in the human body that express the MRGPRX2 receptor include [https://en.wikipedia.org/wiki/Mast_cell mast cells] in the skin, intestines, and trachea <ref name="Porebski">PMID:30619367</ref><ref name="Dondalska">PMID: 33101278</ref>. Mast cells are immune cells responsible for triggering [https://en.wikipedia.org/wiki/Inflammation inflammatory] responses. Mast cells are densely packed with [https://en.wikipedia.org/wiki/Granule_(cell_biology) granules] containing inflammatory chemicals, such as [https://en.wikipedia.org/wiki/Histamine histamine] and [https://en.wikipedia.org/wiki/Heparin heparin]<ref name= "Dondalska" />. Mast cells can be activated by either [https://en.wikipedia.org/wiki/Antibody antibodies] from the immune response or upon [https://en.wikipedia.org/wiki/Ligand ligands] binding to MRGPRX2 receptors on their surface<ref name="McNeil">PMID: 25517090</ref>. Upon activation, mast cells will release histamine-containing granules which can trigger a larger inflammatory response <ref name= "Dondalska" /><ref name="McNeil" />. These responses induce common allergic reaction or [https://en.wikipedia.org/wiki/Anaphylaxis anaphylaxis] symptoms, such as cutaneous itching sensations or airway constriction<ref name= "Cao" /><ref name= "Yang" /><ref name="McNeil">PMID: 25517090</ref>. |
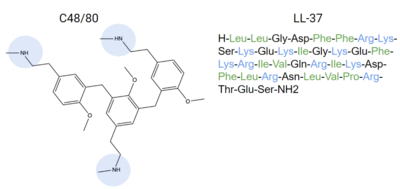
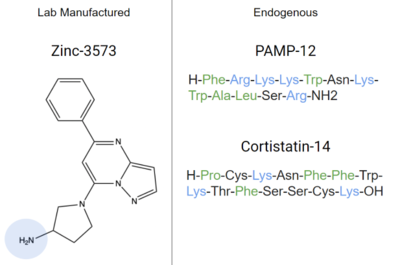
| - | Ligands that bind to MRGPRX2 in the natural environment to produce an allergic response include [https://en.wikipedia.org/wiki/Exogeny#Biology_and_Medicine exogenous] molecules | + | Ligands that bind to MRGPRX2 in the natural environment to produce an allergic response include [https://en.wikipedia.org/wiki/Exogeny#Biology_and_Medicine exogenous] molecules, some being contents of insect venom, molecules like [https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:75295 Compound 48/80 (C48/80)], or other polycationic molecules<ref name= "Dondalska">PMID: 33101278</ref><ref name= "Porebski" />. They can also respond to [https://en.wikipedia.org/wiki/Endogeny_(biology) endogenous] signaling molecules involved in inflammation pathways such as [https://en.wikipedia.org/wiki/Cytokine cytokines], [https://en.wikipedia.org/wiki/Anaphylatoxin anaphylatoxins], or [https://en.wikipedia.org/wiki/Neuropeptide#Receptor_targets neuropeptides]<ref name= "Porebski" />. Many pseudo-allergic drug reactions have been tied to overactivity of MRGPRX2 receptors on mast cells<ref name="McNeil">PMID: 25517090</ref>, so research into receptor-ligand interactions of the MRGPRX2 receptor has the potential to mediate many adverse itching and allergic reaction side effects seen in drugs today<ref name= "McNeil">PMID: 25517090</ref>. |
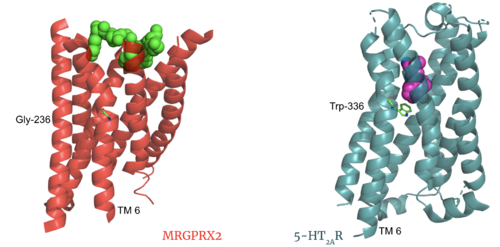
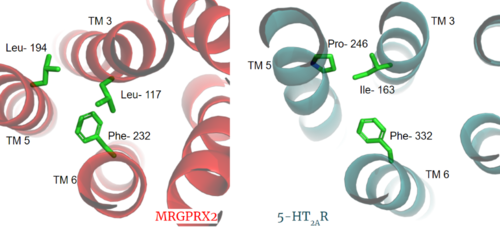
GPCRs are categorized into 6 different classes based on shared sequences and functions. MRGPRX2 is categorized into the [https://proteopedia.org/wiki/index.php/GPCR#Family_A_of_GPCRs Class A] receptor family. However, itch receptors like MGPRX2 have unique structural features from most class A receptors <ref name="Cao">PMID: 34789874</ref><ref name="Yang">PMID: 34789875</ref>. These unique structural features, as seen in '''Class A Family Differences''', cause conformational changes throughout the protein that impact what ligands bind to the receptor<ref name= "Yang" />. | GPCRs are categorized into 6 different classes based on shared sequences and functions. MRGPRX2 is categorized into the [https://proteopedia.org/wiki/index.php/GPCR#Family_A_of_GPCRs Class A] receptor family. However, itch receptors like MGPRX2 have unique structural features from most class A receptors <ref name="Cao">PMID: 34789874</ref><ref name="Yang">PMID: 34789875</ref>. These unique structural features, as seen in '''Class A Family Differences''', cause conformational changes throughout the protein that impact what ligands bind to the receptor<ref name= "Yang" />. | ||
| Line 24: | Line 24: | ||
=== G-Protein === | === G-Protein === | ||
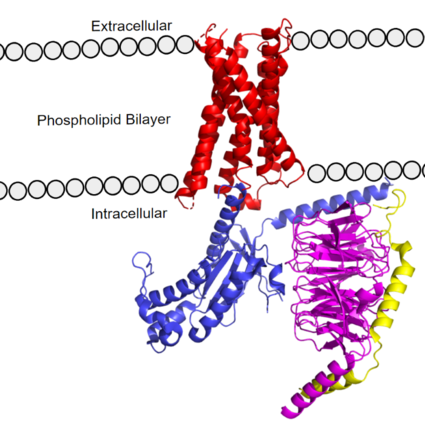
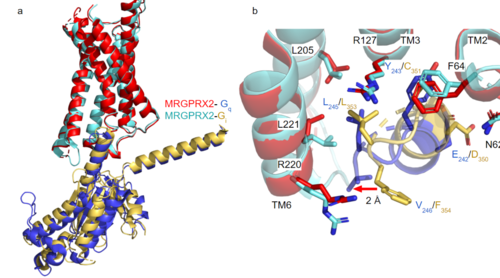
| - | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of <scene name=''90/904305/Structure_overview_alpha/2'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/2'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/2'>gamma</scene> subunits that interact with the intracellular transmembrane region at an <scene name='90/904306/Interface_2/1'>interface</scene> ( '''Figure 2b'''). G-proteins are responsible for | + | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of <scene name=''90/904305/Structure_overview_alpha/2'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/2'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/2'>gamma</scene> subunits that interact with the intracellular transmembrane region at an <scene name='90/904306/Interface_2/1'>interface</scene> ( '''Figure 2b'''). G-proteins are responsible for transmitting extracellular signals into the cell upon activation. Activation leads to a substitution of GDP with GTP within the alpha subunit, causing the alpha subunit to disassociate from the beta and gamma subunits to initiate an intracellular signaling cascade. There are different families of G-alpha subunits, Gαi, Gαs, Gα12/13, and Gαq <ref name="Kamato">PMID: 26664886</ref>. MRGPRX2 binds to both Gαi and Gαq subunits with nearly identical structures despite slightly different amino acids present ( '''Figure 2a''') <ref name= "Cao" /> <ref name= "Yang" />. Throughout this page, MGPRX2 is always shown with Gq. The major difference between the Gq and Gi bound structures comes from one amino acid difference (valine on Gq versus phenylalanine on Gi) that pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. |
[[Image:Gq and gi overlay.png|500px|center|thumb|'''Figure 2a.''' Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow). '''Figure 2b.''' Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. All other interactions are nearly identical.]] | [[Image:Gq and gi overlay.png|500px|center|thumb|'''Figure 2a.''' Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow). '''Figure 2b.''' Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. All other interactions are nearly identical.]] | ||
Revision as of 00:02, 19 April 2022
MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR)
| |||||||||||
References
- ↑ Tuteja N. Signaling through G protein coupled receptors. Plant Signal Behav. 2009 Oct;4(10):942-7. doi: 10.4161/psb.4.10.9530. Epub 2009, Oct 14. PMID:19826234 doi:http://dx.doi.org/10.4161/psb.4.10.9530
- ↑ Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub, 2017 Oct 27. PMID:29075003 doi:http://dx.doi.org/10.1038/nrd.2017.178
- ↑ 3.0 3.1 3.2 Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol. 2018 Dec 20;9:3027. doi: 10.3389/fimmu.2018.03027. eCollection, 2018. PMID:30619367 doi:http://dx.doi.org/10.3389/fimmu.2018.03027
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Dondalska A, Ronnberg E, Ma H, Palsson SA, Magnusdottir E, Gao T, Adam L, Lerner EA, Nilsson G, Lagerstrom M, Spetz AL. Amelioration of Compound 48/80-Mediated Itch and LL-37-Induced Inflammation by a Single-Stranded Oligonucleotide. Front Immunol. 2020 Sep 30;11:559589. doi: 10.3389/fimmu.2020.559589. eCollection, 2020. PMID:33101278 doi:http://dx.doi.org/10.3389/fimmu.2020.559589
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015 Mar 12;519(7542):237-41. doi: 10.1038/nature14022. Epub 2014 Dec 17. PMID:25517090 doi:http://dx.doi.org/10.1038/nature14022
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, Slocum ST, Krumm BE, Lansu K, McCorvy JD, Kroeze WK, English JG, DiBerto JF, Olsen RHJ, Huang XP, Zhang S, Liu Y, Kim K, Karpiak J, Jan LY, Abraham SN, Jin J, Shoichet BK, Fay JF, Roth BL. Structure, function and pharmacology of human itch GPCRs. Nature. 2021 Dec;600(7887):170-175. doi: 10.1038/s41586-021-04126-6. Epub 2021, Nov 17. PMID:34789874 doi:http://dx.doi.org/10.1038/s41586-021-04126-6
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021 Dec;600(7887):164-169. doi: 10.1038/s41586-021-04077-y. Epub 2021, Nov 17. PMID:34789875 doi:http://dx.doi.org/10.1038/s41586-021-04077-y
- ↑ Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, Brimble M, Osman N, Little PJ. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2015 Mar 24;2:14. doi: 10.3389/fcvm.2015.00014. eCollection, 2015. PMID:26664886 doi:http://dx.doi.org/10.3389/fcvm.2015.00014
- ↑ Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs--theoretical and experimental studies. Curr Med Chem. 2012;19(8):1090-109. doi: 10.2174/092986712799320556. PMID:22300046 doi:http://dx.doi.org/10.2174/092986712799320556
- ↑ 10.0 10.1 Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol. 2007 Apr;71(4):959-64. doi: 10.1124/mol.106.029470. Epub 2006 Dec , 27. PMID:17192495 doi:http://dx.doi.org/10.1124/mol.106.029470
- ↑ Naranjo AN, Chevalier A, Cousins GD, Ayettey E, McCusker EC, Wenk C, Robinson AS. Conserved disulfide bond is not essential for the adenosine A2A receptor: Extracellular cysteines influence receptor distribution within the cell and ligand-binding recognition. Biochim Biophys Acta. 2015 Feb;1848(2):603-14. doi: 10.1016/j.bbamem.2014.11.010., Epub 2014 Nov 16. PMID:25445670 doi:http://dx.doi.org/10.1016/j.bbamem.2014.11.010
- ↑ Olivella M, Caltabiano G, Cordomi A. The role of Cysteine 6.47 in class A GPCRs. BMC Struct Biol. 2013 Mar 15;13:3. doi: 10.1186/1472-6807-13-3. PMID:23497259 doi:http://dx.doi.org/10.1186/1472-6807-13-3
- ↑ Gonzalez-Rey E, Chorny A, Robledo G, Delgado M. Cortistatin, a new antiinflammatory peptide with therapeutic effect on lethal endotoxemia. J Exp Med. 2006 Mar 20;203(3):563-71. doi: 10.1084/jem.20052017. Epub 2006 Feb, 21. PMID:16492802 doi:http://dx.doi.org/10.1084/jem.20052017