We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=== Domains === | === Domains === | ||
==== Three Helix Bundle-like Domain ==== | ==== Three Helix Bundle-like Domain ==== | ||
| - | The <scene name='90/904332/Thb-like_domain/1'>Three Helix Bundle-like Domain</scene> mainly has a structural function | + | The <scene name='90/904332/Thb-like_domain/1'>Three Helix Bundle-like Domain</scene> mainly has a structural function as it interacts with the TNF-like domain upon ligand binding. The THB-like domain's α-helix interacts with the helix α-1' and β strand A-1' on the TNF-like domain. This outermost region of the extracellular ligand-binding domain undergoes rigorous structural reorientation upon ligand binding. The THB-like is primarily involved in the dimerization motif of ALK, which dimerizes upon ligand binding. <ref name="Reshetnyak" /> |
==== Poly-Glycine Domain ==== | ==== Poly-Glycine Domain ==== | ||
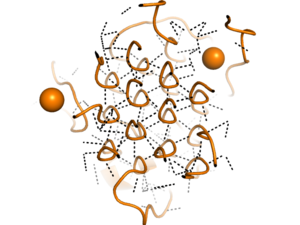
[[Image:glycinehelicesorange.png|300 px|right|thumb|Figure 2. Rare Glycine helices on Anaplastic Lymphoma Kinase]]Located between the THB-like domain and the TNF-like domain, the <scene name='90/904331/Polyg_region1/4'>Poly-Glycine Region</scene> has an important structural role. The GlyR domain also has a rare and unique structure of left-handed glycine helices with hexagonal hydrogen bonding shown in Figure 2. These 14 glycine helices are unique to ALK's function among other tyrosine kinases, as these types of structures on the binding domain are not present. These helices are rigid structures, providing a strong anchor for the ligand binding site while the other domains undergo drastic conformational rearrangements.<ref name="Reshetnyak" /> | [[Image:glycinehelicesorange.png|300 px|right|thumb|Figure 2. Rare Glycine helices on Anaplastic Lymphoma Kinase]]Located between the THB-like domain and the TNF-like domain, the <scene name='90/904331/Polyg_region1/4'>Poly-Glycine Region</scene> has an important structural role. The GlyR domain also has a rare and unique structure of left-handed glycine helices with hexagonal hydrogen bonding shown in Figure 2. These 14 glycine helices are unique to ALK's function among other tyrosine kinases, as these types of structures on the binding domain are not present. These helices are rigid structures, providing a strong anchor for the ligand binding site while the other domains undergo drastic conformational rearrangements.<ref name="Reshetnyak" /> | ||
| Line 23: | Line 23: | ||
==== ALKAL1 ==== | ==== ALKAL1 ==== | ||
<scene name='90/904331/Alkal1/5'>ALKAL1</scene> (Anaplastic Lymphoma Kinase Ligand 1) is a monomeric ligand of ALK, in addition to ALKAL2. Structurally, ALKAL1 and ALKAL2 contain an N-terminal variable region and a conversed C-terminal augmentor domain <ref name="Reshetnyak" />. However, in ALKAL1, this N-terminal variable region is shorter, and shares no similar sequences to ALKAL2. Nevertheless, ALKAL1 shares a 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface which is used in ligand binding <ref name="Reshetnyak" />. | <scene name='90/904331/Alkal1/5'>ALKAL1</scene> (Anaplastic Lymphoma Kinase Ligand 1) is a monomeric ligand of ALK, in addition to ALKAL2. Structurally, ALKAL1 and ALKAL2 contain an N-terminal variable region and a conversed C-terminal augmentor domain <ref name="Reshetnyak" />. However, in ALKAL1, this N-terminal variable region is shorter, and shares no similar sequences to ALKAL2. Nevertheless, ALKAL1 shares a 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface which is used in ligand binding <ref name="Reshetnyak" />. | ||
| - | === Dimerization of | + | === Dimerization of ALK === |
After binding to one of its ligands, ALK undergoes <scene name='90/904331/Alk_full_dimerization/3'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimerization] causes trans-phosphorylation of specific [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residues which in turn amplifies the signal. It has been presumed that the [https://en.wikipedia.org/wiki/Phosphorylation_cascade phosphorylation cascade] activates ALK kinase activity <ref name="Huang" />. | After binding to one of its ligands, ALK undergoes <scene name='90/904331/Alk_full_dimerization/3'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimerization] causes trans-phosphorylation of specific [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residues which in turn amplifies the signal. It has been presumed that the [https://en.wikipedia.org/wiki/Phosphorylation_cascade phosphorylation cascade] activates ALK kinase activity <ref name="Huang" />. | ||
== Function == | == Function == | ||
Revision as of 01:54, 19 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
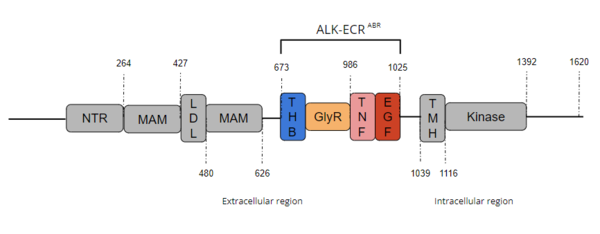
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ 3.0 3.1 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 4.0 4.1 4.2 4.3 Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 5.0 5.1 Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, Ciardiello F, Morgillo F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol Cancer. 2018 Feb 19;17(1):30. doi: 10.1186/s12943-018-0776-2. PMID:29455642 doi:http://dx.doi.org/10.1186/s12943-018-0776-2