We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='7N00' size='350' side='right' caption=' Structure of Anaplastic Lymphoma Kinase [https://www.rcsb.org/structure/7N00 7N00]' scene='90/904331/Alk_full/1'> | <StructureSection load='7N00' size='350' side='right' caption=' Structure of Anaplastic Lymphoma Kinase [https://www.rcsb.org/structure/7N00 7N00]' scene='90/904331/Alk_full/1'> | ||
== Background == | == Background == | ||
| - | Anaplastic Lymphoma Kinase (ALK) is a [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] receptor and a member of the family of [https://proteopedia.org/wiki/index.php/Receptor_tyrosine_kinases Receptor Tyrosine Kinases (RTKs)]. RTKs are a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway. ALK was identified as a novel tyrosine phosphoprotein in 1994 in an analysis of [https://lymphoma.org/aboutlymphoma/nhl/alcl/ Anaplastic Large-Cell Lymphoma], the protein's namesake.<ref name ="Huang" /> A full analysis and characterization of ALK was completed in 1997, properly identifying it as a RTK, and linking it closely to [https://en.wikipedia.org/wiki/Leukocyte_receptor_tyrosine_kinase Leukocyte Tyrosine Kinase] (LTK).<ref name ="Huang" /> | + | Anaplastic Lymphoma Kinase (ALK) is a [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] receptor and a member of the family of [https://proteopedia.org/wiki/index.php/Receptor_tyrosine_kinases Receptor Tyrosine Kinases (RTKs)]. RTKs are a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway. ALK was identified as a novel tyrosine phosphoprotein in 1994 in an analysis of [https://lymphoma.org/aboutlymphoma/nhl/alcl/ Anaplastic Large-Cell Lymphoma], the protein's namesake.<ref name ="Huang" /> A full analysis and characterization of ALK was completed in 1997, properly identifying it as a RTK, and linking it closely to [https://en.wikipedia.org/wiki/Leukocyte_receptor_tyrosine_kinase Leukocyte Tyrosine Kinase] (LTK).<ref name ="Huang" /> ALK's normal activity as a receptor tyrosine kinase is to transfer a gamma-phosphate group from adenosine triphosphate (ATP) to a tyrosine residue on it's substrate.<ref name ="Huang" /> ALK is one of more than 50 RTKs encoded within the human genome, <ref name ="Huang" /> and it's tyrosine kinase activity seems to be especially important in the developing nervous system. <ref name ="Huang" /> |
== Structure == | == Structure == | ||
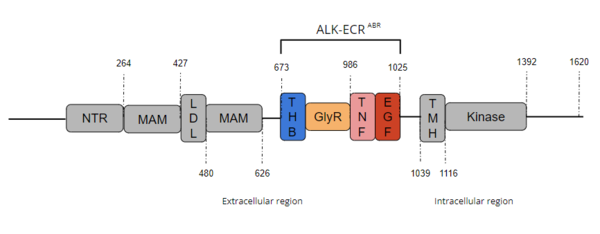
ALK is a close homolog of LTK, and together these two homologues constitute a subgroup within the superfamily of [https://proteopedia.org/wiki/index.php/Insulin_receptor insulin receptors] (IR). ALK is composed of three primary regions: the extracellular region, the transmembrane region, and the intracellular region. [[Image:Full ALK Structure Graphic.PNG|600 px|right|thumb|Figure 1. Overview of Anaplastic Lymphoma Kinase Structure with domains where known structure are color coordinated and other domains are grayed out.]] The extracellular region of ALK contains 8 total domains within 2 fragments. A Three Helix Bundle-like domain (THB-like), a Poly-Glycine domain (GlyR), a Tumor Necrosis Factor-like domain (TNF-like), and an Epidermal Growth Factor-like domain (EGF-like) make up the ligand binding fragment while a N-terminal domain, two [https://en.wikipedia.org/wiki/Meprin_A meprin–A-5] protein–receptor protein tyrosine phosphatase μ (MAM) domains and a [https://en.wikipedia.org/wiki/Low-density_lipoprotein low-density lipoprotein] receptor class A (LDL) domain sandwiched between the two MAM domains make up the second fragment. All four domains of the ligand binding fragment of the extracellular region contribute to ligand-binding <ref name ="Huang" />. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear. The [https://en.wikipedia.org/wiki/Transmembrane_domain transmembrane helical region] (TMH) bridges the gap between the intracellular and extracellular regions. The intracellular tyrosine kinase domain features the Kinase domain and the C-terminal end (Figure 1). | ALK is a close homolog of LTK, and together these two homologues constitute a subgroup within the superfamily of [https://proteopedia.org/wiki/index.php/Insulin_receptor insulin receptors] (IR). ALK is composed of three primary regions: the extracellular region, the transmembrane region, and the intracellular region. [[Image:Full ALK Structure Graphic.PNG|600 px|right|thumb|Figure 1. Overview of Anaplastic Lymphoma Kinase Structure with domains where known structure are color coordinated and other domains are grayed out.]] The extracellular region of ALK contains 8 total domains within 2 fragments. A Three Helix Bundle-like domain (THB-like), a Poly-Glycine domain (GlyR), a Tumor Necrosis Factor-like domain (TNF-like), and an Epidermal Growth Factor-like domain (EGF-like) make up the ligand binding fragment while a N-terminal domain, two [https://en.wikipedia.org/wiki/Meprin_A meprin–A-5] protein–receptor protein tyrosine phosphatase μ (MAM) domains and a [https://en.wikipedia.org/wiki/Low-density_lipoprotein low-density lipoprotein] receptor class A (LDL) domain sandwiched between the two MAM domains make up the second fragment. All four domains of the ligand binding fragment of the extracellular region contribute to ligand-binding <ref name ="Huang" />. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear. The [https://en.wikipedia.org/wiki/Transmembrane_domain transmembrane helical region] (TMH) bridges the gap between the intracellular and extracellular regions. The intracellular tyrosine kinase domain features the Kinase domain and the C-terminal end (Figure 1). | ||
| Line 26: | Line 26: | ||
After binding to one of its ligands, ALK undergoes <scene name='90/904331/Alk_full_dimerization/3'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimerization] causes trans-phosphorylation of specific [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residues which in turn amplifies the signal. It has been presumed that the [https://en.wikipedia.org/wiki/Phosphorylation_cascade phosphorylation cascade] activates ALK kinase activity <ref name="Huang" />. | After binding to one of its ligands, ALK undergoes <scene name='90/904331/Alk_full_dimerization/3'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimerization] causes trans-phosphorylation of specific [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residues which in turn amplifies the signal. It has been presumed that the [https://en.wikipedia.org/wiki/Phosphorylation_cascade phosphorylation cascade] activates ALK kinase activity <ref name="Huang" />. | ||
== Function == | == Function == | ||
| - | ALK plays a role in [https://en.wikipedia.org/wiki/Cell_signaling cellular communication] and in the normal development and function of the [https://en.wikipedia.org/wiki/Nervous_system nervous system] It is present largely in the developing nervous system of a fetus and newborn, and overtime the expression of ALK dwindles with age. In addition to being expressed heavily in the brain, ALK has been shown to be present in the small intestine, testis, prostate, and colon <ref name="Della Corte">PMID:29455642</ref>. | + | ALK plays a role in [https://en.wikipedia.org/wiki/Cell_signaling cellular communication] and in the normal development and function of the [https://en.wikipedia.org/wiki/Nervous_system nervous system]<ref name ="Huang" /> It is present largely in the developing nervous system of a fetus and newborn, and overtime the expression of ALK dwindles with age.<ref name ="Huang" /> In addition to being expressed heavily in the brain, ALK has been shown to be present in the small intestine, testis, prostate, and colon <ref name="Della Corte">PMID:29455642</ref>. |
== Disease and Medical Relevance == | == Disease and Medical Relevance == | ||
=== Cancer === | === Cancer === | ||
Revision as of 02:25, 19 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ 3.0 3.1 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 4.0 4.1 4.2 4.3 Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 5.0 5.1 Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, Ciardiello F, Morgillo F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol Cancer. 2018 Feb 19;17(1):30. doi: 10.1186/s12943-018-0776-2. PMID:29455642 doi:http://dx.doi.org/10.1186/s12943-018-0776-2