We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1723
From Proteopedia
(Difference between revisions)
| Line 36: | Line 36: | ||
==== ''DRY Motif'' ==== | ==== ''DRY Motif'' ==== | ||
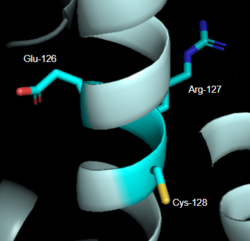
| - | The DRY motif is a proton microswitch that is located near the G-protein binding site C-terminal on TM3 <ref name="Schonegge"/>. It acts as an ion lock when the GPCR is not being activated, preventing unnecessary activation of the G-proteins <ref name="Zhou"/>. This motif is conserved in typical Class A GPCRS however, in MRGPRX2 it is only partially conserved. The arginine is conserved, while the aspartate is replaced by a glutamate and the tyrosine is replaced by a cysteine <ref name="Yang"/> <ref name="Sandoval">Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004 </ref>. The replacement of tyrosine for cysteine results in the helices coming closer together, creating a shallower binding pocket.<ref name="Zhou"/> | + | The DRY motif is a proton microswitch that is located near the G-protein binding site C-terminal on TM3 <ref name="Schonegge"/>. It acts as an ion lock when the GPCR is not being activated, preventing unnecessary activation of the G-proteins <ref name="Zhou"/>. This motif is conserved in typical Class A GPCRS however, in MRGPRX2 it is only partially conserved. The arginine is conserved, while the aspartate is replaced by a glutamate and the tyrosine is replaced by a cysteine <ref name="Yang"/> <ref name="Sandoval">Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004 </ref> (Figure 2). The replacement of tyrosine for cysteine results in the helices coming closer together, creating a shallower binding pocket.<ref name="Zhou"/> |
==== ''Sodium Binding'' ==== | ==== ''Sodium Binding'' ==== | ||
| Line 46: | Line 46: | ||
=== 1. Binding Pocket === | === 1. Binding Pocket === | ||
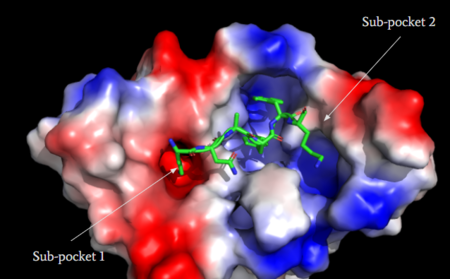
| - | MRGPRX2 consists of two binding pockets (seen in Figure | + | MRGPRX2 consists of two binding pockets (seen in Figure 3). Sub-pocket 1 consists of acidic catalytic residues Asp-184 and Glu-164 that interact with substrates by making ion pairs. There are also some hydrophobic aromatic residues, Phe-170, Trp-243, and Phe-244, towards the top of the binding pocket.<ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref> These residues provide stabilization with ligands through stacking. Lastly, this pocket is in close proximity with the commonly conserved disulfide bond (formed by Cys-168 and Cys-180) seen in most Class A GPCRs. The second binding pocket forms hydrophobic interactions with larger substrates (seen in Figure 2), but is generally less studied.<ref name="Cao"/> |
| - | [[Image:Electro.PNG|450px|center|thumb|'''Figure | + | [[Image:Electro.PNG|450px|center|thumb|'''Figure 3''': Binding pocket of MRGPRX2 with cortistatin-14. Two different binding pockets are present in MRGPRX2 and cortistatin-14 interacts with both of them. <ref name="Cao"/>]] |
==== Agonists ==== | ==== Agonists ==== | ||
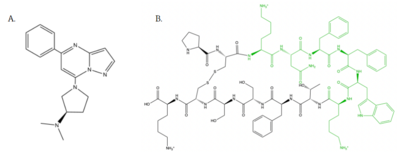
| - | [[Image:Agoniststogether.PNG |400px|right|thumb|'''Figure | + | [[Image:Agoniststogether.PNG |400px|right|thumb|'''Figure 4''': Structure of MRGPRX2 Agonists. (A) Structure of R-Zinc 3573. A cationic ligand selected for binding to MRGPRX2.<ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref> (B) Structure of Cortistatin-14 with resolved amino acids highlighted in green. These ligands were used as a probes for MRGPRX2 function and stabilization for structure determination.<ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>]] |
===== Small Molecule ===== | ===== Small Molecule ===== | ||
| - | <scene name='90/904328/Zinc/4'>(R)-Zinc-3573</scene> is a cation ligand that selectively binds to MRGPRX2 (Figure | + | <scene name='90/904328/Zinc/4'>(R)-Zinc-3573</scene> is a cation ligand that selectively binds to MRGPRX2 (Figure 4). Its N-dimethyl is inserted into subpocket 1 of the binding cavity with aromatic amino acid residues Phe-170, Trp-243, and Phe-244. In this <scene name='90/904328/Zizwithaa/5'>cavity</scene>, N-dimethyl group on the ligand makes ion pairs with Asp-184 and Glu-164. The ligand is then stabilized by stacking its ring with Trp-248 and the Cys-168 to Cys-180 disulfide bond <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>. |
===== Peptide ===== | ===== Peptide ===== | ||
| - | <scene name='90/904328/Overview_x2_c_pt_3/4'>Cortistatin-14</scene> is one of the peptide ligands that binds to MRGPRX2 (Figure | + | <scene name='90/904328/Overview_x2_c_pt_3/4'>Cortistatin-14</scene> is one of the peptide ligands that binds to MRGPRX2 (Figure 4). Cortistatin-14 interacts with the binding pocket through an <scene name='90/904328/Zic14_pt_3/5'>electrostatic</scene> interaction in sub-pocket 1 between Lys-3 on the peptide and Glu-164 and Asp-184 on MRGPRX2 <ref name="Cao"/>. Additionally, there are hydrophobic interactions in sub-pocket 2 between the peptide and the binding pocket due to the large hydrophobic amino acids on Cortistatin-14. |
=== 2. MRGPRX2 interaction with G-Protein === | === 2. MRGPRX2 interaction with G-Protein === | ||
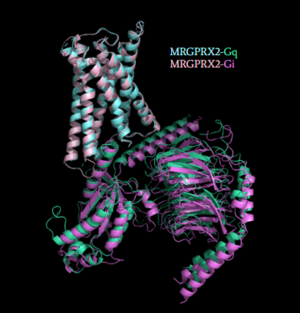
| - | [[Image:Gq_and_Gi_snip.PNG|300px|right|thumb|'''Figure | + | [[Image:Gq_and_Gi_snip.PNG|300px|right|thumb|'''Figure 5''': Comparison of the conformational change for MRGPRX2 (light blue) coupled with Gq (teal) and MRGPRX2 (pink) coupled with Gi (purple).<ref name="Cao"/> PDB entry [https://www.rcsb.org/structure/7S8N 7S8N] and [https://www.rcsb.org/structure/7S8O 7S8O]]] |
| - | Once the ligand is bound, MRGPRX2 undergoes a conformational change that is transmitted through to the [https://en.wikipedia.org/wiki/G_protein#:~:text=G%20proteins%2C%20also%20known%20as,a%20cell%20to%20its%20interior. G-protein]. This conformational change is affected by the aforementioned deviances from Class A GPCRs. Rather than a large conformational change, a subtle one is induced. This will allow MRGPRX2 to interact with a G-protein. <scene name='90/904327/Gproteins/2'>G-proteins</scene> are composed of 3 subunits; α, β, and γ. When activated, the receptor acts as a Guanine nucleotide factor (GEF) which will allow the Gα subunit to have its GDP be replaced by a GTP. This will cause the Gα subunit to dissociate from the dimer Gβγ. The Gα subunit is then able to act as a secondary messenger to begin the signal transduction in the cell. MRGPRX2 interacts with two different types of G-proteins; Gi and Gq. These G-proteins are activated by similar interactions with the receptor however, they are structurally different and changes in the induced conformation are observed (Figure | + | Once the ligand is bound, MRGPRX2 undergoes a conformational change that is transmitted through to the [https://en.wikipedia.org/wiki/G_protein#:~:text=G%20proteins%2C%20also%20known%20as,a%20cell%20to%20its%20interior. G-protein]. This conformational change is affected by the aforementioned deviances from Class A GPCRs. Rather than a large conformational change, a subtle one is induced. This will allow MRGPRX2 to interact with a G-protein. <scene name='90/904327/Gproteins/2'>G-proteins</scene> are composed of 3 subunits; α, β, and γ. When activated, the receptor acts as a Guanine nucleotide factor (GEF) which will allow the Gα subunit to have its GDP be replaced by a GTP. This will cause the Gα subunit to dissociate from the dimer Gβγ. The Gα subunit is then able to act as a secondary messenger to begin the signal transduction in the cell. MRGPRX2 interacts with two different types of G-proteins; Gi and Gq. These G-proteins are activated by similar interactions with the receptor however, they are structurally different and changes in the induced conformation are observed (Figure 5). |
=== 3.After G-Protein Activation === | === 3.After G-Protein Activation === | ||
| Line 75: | Line 75: | ||
== Clinical Relevance == | == Clinical Relevance == | ||
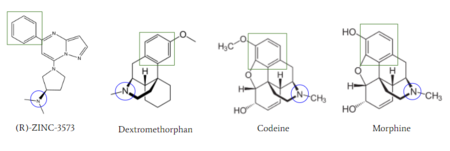
| - | [[Image:Zinc_and_drugs_snip.PNG|450px|right|thumb|'''Figure | + | [[Image:Zinc_and_drugs_snip.PNG|450px|right|thumb|'''Figure 6''': Structures of (R)-Zinc-3573, Dextromethorphan, Morphine, and Codeine. The blue circles indicate conserved basic N-dimethyl group and the green squares show the conserved benzene rings.<ref name="Cao"/>]] |
| - | MRGPRX2 activation is associated with chronic itching or anaphylaxis reactions, a common side effect of many prescribed medications. <ref name="Cao"/> Among these drugs are opiods [https://en.wikipedia.org/wiki/Morphine morphine] and [https://en.wikipedia.org/wiki/Codeine codeine] and [https://en.wikipedia.org/wiki/Dextromethorphan dextromethorphan]. By analyzing the structures of these drugs, it can be seen that they contain chemical features similar to <scene name='90/904328/Zizwithaa/5'>agonist R-Zinc-3573</scene> (see Figure | + | MRGPRX2 activation is associated with chronic itching or anaphylaxis reactions, a common side effect of many prescribed medications. <ref name="Cao"/> Among these drugs are opiods [https://en.wikipedia.org/wiki/Morphine morphine] and [https://en.wikipedia.org/wiki/Codeine codeine] and [https://en.wikipedia.org/wiki/Dextromethorphan dextromethorphan]. By analyzing the structures of these drugs, it can be seen that they contain chemical features similar to <scene name='90/904328/Zizwithaa/5'>agonist R-Zinc-3573</scene> (see Figure 6). They contain a conserved benzene ring that stabilizes them in binding pocket one (see Figure 6). Similarly, they contain an N-dimethyl group that would allow them to form key bonds with residues Asp-184 and Glu-164. Lastly, these drugs have a similar shape and size to that of the agonist R-Zinc-3573.<ref name="Babina"> Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017</ref> These structural and chemical similarities indicate the possibility of a similar binding mechanism. |
This receptor is shallow and is able to bind to a variety of drugs, creating a need a solution to mediate the effects of MRGPRX2 activation. Currently, there is research for the development of small antagonists that will induce an anti-inflammatory effect by prevent [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE-dependent] mast cell degranulation.<ref name="McNeil">McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full</ref> There are two antagonists that have been adapted from being agonists to another Class A GPCR, [https://en.wikipedia.org/wiki/NK1_receptor_antagonist neurokinin-1 receptor (NK-1R)].<ref name="Ogasawara">Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R</ref> Through the development of this research, these adverse side effects can be relieved and improve patient satisfaction. | This receptor is shallow and is able to bind to a variety of drugs, creating a need a solution to mediate the effects of MRGPRX2 activation. Currently, there is research for the development of small antagonists that will induce an anti-inflammatory effect by prevent [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE-dependent] mast cell degranulation.<ref name="McNeil">McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full</ref> There are two antagonists that have been adapted from being agonists to another Class A GPCR, [https://en.wikipedia.org/wiki/NK1_receptor_antagonist neurokinin-1 receptor (NK-1R)].<ref name="Ogasawara">Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R</ref> Through the development of this research, these adverse side effects can be relieved and improve patient satisfaction. | ||
Revision as of 23:21, 20 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
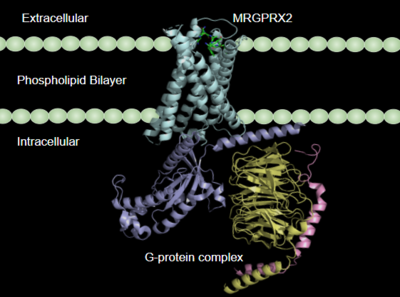
Human Itch Mas-Related G-Protein Coupled Receptor
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Cao, Can, et al. "Structure, function and pharmacology of human itch GPCRs." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04126-6
- ↑ Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z
- ↑ 3.0 3.1 Zhang D, Zhao Q, Wu B. Structural Studies of G Protein-Coupled Receptors. Mol Cells. 2015 Oct;38(10):836-42. doi: 10.14348/molcells.2015.0263. Epub 2015, Oct 15. PMID:26467290 doi:http://dx.doi.org/10.14348/molcells.2015.0263
- ↑ 4.0 4.1 4.2 Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife. 2019 Dec 19;8. pii: 50279. doi: 10.7554/eLife.50279. PMID:31855179 doi:http://dx.doi.org/10.7554/eLife.50279
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y
- ↑ 6.0 6.1 Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x
- ↑ Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017
- ↑ McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full
- ↑ Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R