We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
==== Three Helix Bundle-like Domain ==== | ==== Three Helix Bundle-like Domain ==== | ||
The <scene name='90/904332/Thb-like_domain/1'>Three Helix Bundle-like Domain</scene> performs a structural function by interacting with the TNF-like domain upon ligand binding.<ref name="Reshetnyak" /> The THB-like domain's α-helix interacts with the helix α-1' and β strand A-1' on the TNF-like domain.<ref name="Reshetnyak" /> This outermost region of the extracellular ligand-binding domain undergoes substantial structural reorientation upon ligand binding.<ref name="Reshetnyak" /> The THB-like is primarily involved in the dimerization motif of ALK, which dimerizes upon ligand binding. <ref name="Reshetnyak" /> | The <scene name='90/904332/Thb-like_domain/1'>Three Helix Bundle-like Domain</scene> performs a structural function by interacting with the TNF-like domain upon ligand binding.<ref name="Reshetnyak" /> The THB-like domain's α-helix interacts with the helix α-1' and β strand A-1' on the TNF-like domain.<ref name="Reshetnyak" /> This outermost region of the extracellular ligand-binding domain undergoes substantial structural reorientation upon ligand binding.<ref name="Reshetnyak" /> The THB-like is primarily involved in the dimerization motif of ALK, which dimerizes upon ligand binding. <ref name="Reshetnyak" /> | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
==== Poly-Glycine Domain ==== | ==== Poly-Glycine Domain ==== | ||
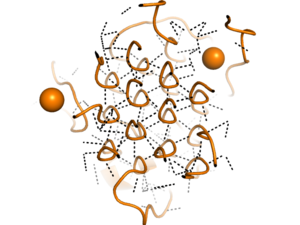
[[Image:glycinehelicesorange.png|300 px|right|thumb|Figure 2. Rare Glycine helices on Anaplastic Lymphoma Kinase; The structure of extracellular ALK is shown in a perpendicular, cross sectional way, highlighted in orange. In black, hydrogen bonds are structured in a hexagonal-like way. Made using [https://www.rcsb.org/structure/7N00 7N00]]]Located between the THB-like domain and the TNF-like domain, the <scene name='90/904331/Polyg_region1/4'>Poly-Glycine Region</scene> has an important structural role.<ref name="Reshetnyak" /> The GlyR domain also has a rare and unique structure of left-handed glycine helices with hexagonal hydrogen bonding (Figure 2).<ref name="Reshetnyak" /> These 14 glycine helices are unique to ALK's function among other tyrosine kinases.<ref name="Reshetnyak" /> These helices are rigid structures, providing a strong anchor for the ligand binding site while the other domains undergo conformational rearrangements.<ref name="Reshetnyak" /> | [[Image:glycinehelicesorange.png|300 px|right|thumb|Figure 2. Rare Glycine helices on Anaplastic Lymphoma Kinase; The structure of extracellular ALK is shown in a perpendicular, cross sectional way, highlighted in orange. In black, hydrogen bonds are structured in a hexagonal-like way. Made using [https://www.rcsb.org/structure/7N00 7N00]]]Located between the THB-like domain and the TNF-like domain, the <scene name='90/904331/Polyg_region1/4'>Poly-Glycine Region</scene> has an important structural role.<ref name="Reshetnyak" /> The GlyR domain also has a rare and unique structure of left-handed glycine helices with hexagonal hydrogen bonding (Figure 2).<ref name="Reshetnyak" /> These 14 glycine helices are unique to ALK's function among other tyrosine kinases.<ref name="Reshetnyak" /> These helices are rigid structures, providing a strong anchor for the ligand binding site while the other domains undergo conformational rearrangements.<ref name="Reshetnyak" /> | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
==== Tumor-Necrosis Factor-like Domain ==== | ==== Tumor-Necrosis Factor-like Domain ==== | ||
The <scene name='90/904331/Tnf-like_domain/2'>Tumor Necrosis Factor-like Domain</scene> interacts with the THB-like domain to begin the conformational changes associated with ligand binding.<ref name="Reshetnyak" /> Located in approximately the midregion of the extracellular region, the TNF-like domain bridges the gap between the GlyR domain and the EGF-like domain. The TNF-like domain also assists in mediating ligand binding with the EGF-like domain<ref name="Reshetnyak" /> by interacting with the THB-like domain to facilitate the critical conformation changes required for dimerization and ligand recognition.<ref name="Reshetnyak" /> | The <scene name='90/904331/Tnf-like_domain/2'>Tumor Necrosis Factor-like Domain</scene> interacts with the THB-like domain to begin the conformational changes associated with ligand binding.<ref name="Reshetnyak" /> Located in approximately the midregion of the extracellular region, the TNF-like domain bridges the gap between the GlyR domain and the EGF-like domain. The TNF-like domain also assists in mediating ligand binding with the EGF-like domain<ref name="Reshetnyak" /> by interacting with the THB-like domain to facilitate the critical conformation changes required for dimerization and ligand recognition.<ref name="Reshetnyak" /> | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
==== Epidermal Growth Factor-like Domain ==== | ==== Epidermal Growth Factor-like Domain ==== | ||
Unlike the poly-Glycine helices, the <scene name='90/904331/Egf_like_domain/3'>Epidermal Growth Factor-like Domain</scene> is malleable and repositioning of this domain is essential for activation of the protein.<ref name="Reshetnyak" /> This domain undergoes conformational changes upon ligand binding and when in contact with the TNF-like domain.<ref name="Reshetnyak" /> The interface between the EGF-like and TNF-like domains are primarily hydrophobic residues, which enables their flexibility with regards to one another.<ref name="Reshetnyak" /> Major motifs in the EGF-like domain are major and minor β-hairpins, which are stabilized by 3 conserved disulfide bridges. <ref name="Reshetnyak" /> | Unlike the poly-Glycine helices, the <scene name='90/904331/Egf_like_domain/3'>Epidermal Growth Factor-like Domain</scene> is malleable and repositioning of this domain is essential for activation of the protein.<ref name="Reshetnyak" /> This domain undergoes conformational changes upon ligand binding and when in contact with the TNF-like domain.<ref name="Reshetnyak" /> The interface between the EGF-like and TNF-like domains are primarily hydrophobic residues, which enables their flexibility with regards to one another.<ref name="Reshetnyak" /> Major motifs in the EGF-like domain are major and minor β-hairpins, which are stabilized by 3 conserved disulfide bridges. <ref name="Reshetnyak" /> | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
== Extracellular Domain Binding == | == Extracellular Domain Binding == | ||
=== Ligands === | === Ligands === | ||
| Line 24: | Line 24: | ||
==== ALKAL2 ==== | ==== ALKAL2 ==== | ||
<scene name='90/904331/Alkal2/3'>ALKAL2</scene> is a shared ligand of ALK and LTK. Dimeric ALKAL2 and monomeric ALKAL2-AD both induce dimerization of ALK <ref name="Reshetnyak">PMID:34819673</ref>. Structurally, ALKAL2 has an N-terminal variable region, a conserved augmentor domain, and tends to aggregate in the cell <ref name="Reshetnyak" />. Overexpression of ALKAL2 is linked to high-risk [https://en.wikipedia.org/wiki/Neuroblastoma neuroblastoma] in absence of an ALK mutation <ref name="Borenas">PMID:33411331</ref>. | <scene name='90/904331/Alkal2/3'>ALKAL2</scene> is a shared ligand of ALK and LTK. Dimeric ALKAL2 and monomeric ALKAL2-AD both induce dimerization of ALK <ref name="Reshetnyak">PMID:34819673</ref>. Structurally, ALKAL2 has an N-terminal variable region, a conserved augmentor domain, and tends to aggregate in the cell <ref name="Reshetnyak" />. Overexpression of ALKAL2 is linked to high-risk [https://en.wikipedia.org/wiki/Neuroblastoma neuroblastoma] in absence of an ALK mutation <ref name="Borenas">PMID:33411331</ref>. | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
==== ALKAL1 ==== | ==== ALKAL1 ==== | ||
<scene name='90/904331/Alkal1/5'>ALKAL1</scene> is a monomeric ligand of ALK. Structurally, ALKAL1 shares the same architecture as ALKAL2 with an N-terminal variable region and a conserved C-terminal augmentor domain <ref name="Reshetnyak" />. However, in ALKAL1, the N-terminal variable region is shorter, and has limited sequence similarity to ALKAL2. Overall, ALKAL1 still shares 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface for ligand binding <ref name="Reshetnyak" />. ALKAL1 as a monomer, however, binds to ALK with poor stability<ref name ="Chen">PMID:33391411</ref> and was only found to stimulate ALK dimerization at much higher concentrations than ALKAL2.<ref name="Reshetnyak2">PMID:26630010</ref> | <scene name='90/904331/Alkal1/5'>ALKAL1</scene> is a monomeric ligand of ALK. Structurally, ALKAL1 shares the same architecture as ALKAL2 with an N-terminal variable region and a conserved C-terminal augmentor domain <ref name="Reshetnyak" />. However, in ALKAL1, the N-terminal variable region is shorter, and has limited sequence similarity to ALKAL2. Overall, ALKAL1 still shares 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface for ligand binding <ref name="Reshetnyak" />. ALKAL1 as a monomer, however, binds to ALK with poor stability<ref name ="Chen">PMID:33391411</ref> and was only found to stimulate ALK dimerization at much higher concentrations than ALKAL2.<ref name="Reshetnyak2">PMID:26630010</ref> | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
=== Binding Site === | === Binding Site === | ||
This site doesn't start out surrounding the [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand], instead the ligand binding initiates [https://en.wikipedia.org/wiki/Conformational_change conformational changes] across the protein. The ligands for ALK have highly positively charged faces that interact with the TNF-like region, the primary ligand-binding site on the extracellular region<ref name="Li" />. [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) Salt bridges] between the positively charged residues on the ligand and negatively charged residues on the receptor are stabilized by ligand binding. Three of these <scene name='90/904331/Salt_bridge_overview/1'>salt bridges</scene> occur between <scene name='90/904331/Salt_bridge_859_140/3'>E859 and R140</scene>, <scene name='90/904331/Salt_bridge_974_136/4'>E974 and R136</scene>, and <scene name='90/904331/Salt_bridge_978_123_133/3'>E978 with both R123 and R133</scene>. These strong ionic interactions also induce the conformational changes in the extracellular domain that induce the signaling pathway. <ref name="Reshetnyak" /> | This site doesn't start out surrounding the [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand], instead the ligand binding initiates [https://en.wikipedia.org/wiki/Conformational_change conformational changes] across the protein. The ligands for ALK have highly positively charged faces that interact with the TNF-like region, the primary ligand-binding site on the extracellular region<ref name="Li" />. [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) Salt bridges] between the positively charged residues on the ligand and negatively charged residues on the receptor are stabilized by ligand binding. Three of these <scene name='90/904331/Salt_bridge_overview/1'>salt bridges</scene> occur between <scene name='90/904331/Salt_bridge_859_140/3'>E859 and R140</scene>, <scene name='90/904331/Salt_bridge_974_136/4'>E974 and R136</scene>, and <scene name='90/904331/Salt_bridge_978_123_133/3'>E978 with both R123 and R133</scene>. These strong ionic interactions also induce the conformational changes in the extracellular domain that induce the signaling pathway. <ref name="Reshetnyak" /> | ||
| - | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | + | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' |
=== Dimerization of ALK === | === Dimerization of ALK === | ||
After binding to one of its ligands, ALK undergoes <scene name='90/904331/Alk_full_dimerization/3'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimerization] causes trans-phosphorylation of specific [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residues which in turn amplifies the signal. It has been presumed that the [https://en.wikipedia.org/wiki/Phosphorylation_cascade phosphorylation cascade] activates ALK kinase activity <ref name="Huang" />. | After binding to one of its ligands, ALK undergoes <scene name='90/904331/Alk_full_dimerization/3'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimerization] causes trans-phosphorylation of specific [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residues which in turn amplifies the signal. It has been presumed that the [https://en.wikipedia.org/wiki/Phosphorylation_cascade phosphorylation cascade] activates ALK kinase activity <ref name="Huang" />. | ||
Revision as of 02:36, 21 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
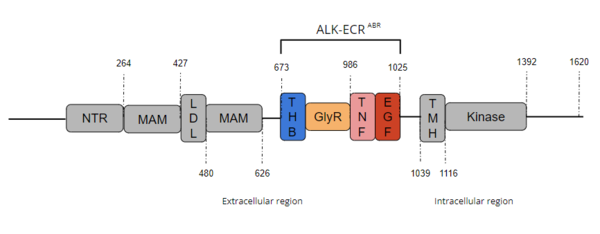
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997 Jan 30;14(4):439-49. doi: 10.1038/sj.onc.1200849. PMID:9053841 doi:http://dx.doi.org/10.1038/sj.onc.1200849
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 3.0 3.1 3.2 Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, Ciardiello F, Morgillo F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol Cancer. 2018 Feb 19;17(1):30. doi: 10.1186/s12943-018-0776-2. PMID:29455642 doi:http://dx.doi.org/10.1186/s12943-018-0776-2
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ 5.0 5.1 5.2 5.3 Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 6.0 6.1 6.2 Chen S, Wang B, Fu X, Liang Y, Chai X, Ye Z, Li R, He Y, Kong G, Lian J, Li X, Chen T, Zhang X, Qiu X, Tang X, Zhou K, Lin B, Zeng J. ALKAL1 gene silencing prevents colorectal cancer progression via suppressing Sonic Hedgehog (SHH) signaling pathway. J Cancer. 2021 Jan 1;12(1):150-162. doi: 10.7150/jca.46447. eCollection 2021. PMID:33391411 doi:http://dx.doi.org/10.7150/jca.46447
- ↑ Reshetnyak AV, Murray PB, Shi X, Mo ES, Mohanty J, Tome F, Bai H, Gunel M, Lax I, Schlessinger J. Augmentor alpha and beta (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):15862-7. doi:, 10.1073/pnas.1520099112. Epub 2015 Nov 16. PMID:26630010 doi:http://dx.doi.org/10.1073/pnas.1520099112
- ↑ 8.0 8.1 8.2 8.3 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 9.0 9.1 Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, Chow AK, Weiser DA, Belcastro LT, Winter C, Bresler SC, Vigny M, Mazot P, Asgharzadeh S, Seeger RC, Zhao H, Guo R, Christensen JG, Orange JS, Pawel BR, Lemmon MA, Mosse YP. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012 Nov 15;31(46):4859-67. doi: 10.1038/onc.2011.647. Epub 2012 Jan, 23. PMID:22266870 doi:http://dx.doi.org/10.1038/onc.2011.647