We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=== Known Extracellular Domains === | === Known Extracellular Domains === | ||
==== Three Helix Bundle-like Domain ==== | ==== Three Helix Bundle-like Domain ==== | ||
| - | The <scene name='90/904332/Thb-like_domain/1'>Three Helix Bundle-like Domain</scene> performs a structural function by interacting with the TNF-like domain upon ligand binding.<ref name="Reshetnyak" /> The THB-like domain's α-helix interacts with the helix α-1' and β strand A-1' on the TNF-like domain.<ref name="Reshetnyak" /> This outermost region of the extracellular ligand-binding domain undergoes substantial structural reorientation upon ligand binding.<ref name="Reshetnyak" /> The THB-like is primarily involved in the <scene name='90/904332/Thb-like_tnf-like_interface/1'>dimerization motif</scene> of ALK, which | + | The <scene name='90/904332/Thb-like_domain/1'>Three Helix Bundle-like Domain</scene> performs a structural function by interacting with the TNF-like domain upon ligand binding.<ref name="Reshetnyak" /> The THB-like domain's α-helix interacts with the helix α-1' and β strand A-1' on the TNF-like domain.<ref name="Reshetnyak" /> This outermost region of the extracellular ligand-binding domain undergoes substantial structural reorientation upon ligand binding.<ref name="Reshetnyak" /> The THB-like is primarily involved in the <scene name='90/904332/Thb-like_tnf-like_interface/1'>dimerization motif</scene> of ALK and interacts with the TNF-like domain, which causes dimerization of ALK. <ref name="Reshetnyak" /> |
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
==== Poly-Glycine Domain ==== | ==== Poly-Glycine Domain ==== | ||
| Line 26: | Line 26: | ||
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
==== ALKAL1 ==== | ==== ALKAL1 ==== | ||
| - | <scene name='90/904331/Alkal1/5'>ALKAL1</scene> is a monomeric ligand of ALK. Structurally, ALKAL1 shares the same architecture as ALKAL2 with an N-terminal variable region and a conserved C-terminal augmentor domain <ref name="Reshetnyak" />. However, in ALKAL1, the N-terminal variable region is shorter, and has limited sequence similarity to ALKAL2. Overall, ALKAL1 still shares 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface for ligand binding <ref name="Reshetnyak" />. ALKAL1 as a monomer, however, binds to ALK with poor stability<ref name ="Chen">PMID:33391411</ref> and was only found to stimulate ALK dimerization at much higher concentrations than ALKAL2.<ref name="Reshetnyak2">PMID:26630010</ref> | + | <scene name='90/904331/Alkal1/5'>ALKAL1</scene> is a monomeric ligand of ALK. Structurally, ALKAL1 shares the same architecture as ALKAL2 with an N-terminal variable region and a conserved C-terminal augmentor domain <ref name="Reshetnyak" />. However, in ALKAL1, the N-terminal variable region is shorter, and has limited sequence similarity to ALKAL2. Overall, ALKAL1 still shares 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface for ligand binding to the TNF-like domain<ref name="Reshetnyak" />. ALKAL1 as a monomer, however, binds to ALK with poor stability<ref name ="Chen">PMID:33391411</ref> and was only found to stimulate ALK dimerization at much higher concentrations than ALKAL2.<ref name="Reshetnyak2">PMID:26630010</ref> |
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
=== Binding Site === | === Binding Site === | ||
| - | The binding site is located on the TNF-like region. <ref name="DeMunck">PMID:34646012</ref> This site doesn't start out surrounding the [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand], instead the ligand binding initiates [https://en.wikipedia.org/wiki/Conformational_change conformational changes] across the protein. The ligands for ALK have highly positively charged faces that interact with the TNF-like region, the primary ligand-binding site on the extracellular region<ref name="Li" />. [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) Salt bridges] between the positively charged residues on the ligand and negatively charged residues on the receptor are stabilized by ligand binding. Three of these <scene name='90/904331/Salt_bridge_overview/1'>salt bridges</scene> occur between <scene name='90/904331/Salt_bridge_859_140/3'>E859 and R140</scene>, <scene name='90/904331/Salt_bridge_974_136/4'>E974 and R136</scene>, and <scene name='90/904331/Salt_bridge_978_123_133/3'>E978 with both R123 and R133</scene>. These strong ionic interactions also induce the conformational changes in the extracellular domain that induce the signaling pathway. <ref name="Reshetnyak" /> | + | The binding site is located on the TNF-like region. <ref name="DeMunck">PMID:34646012</ref> This site doesn't start out surrounding the [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand], instead the ligand binding initiates [https://en.wikipedia.org/wiki/Conformational_change conformational changes] across the extracellular region of the protein. The ligands for ALK have highly positively charged faces that interact with the TNF-like region, the primary ligand-binding site on the extracellular region<ref name="Li" />. [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) Salt bridges] between the positively charged residues on the ligand and negatively charged residues on the receptor are stabilized by ligand binding. Three of these <scene name='90/904331/Salt_bridge_overview/1'>salt bridges</scene> occur between <scene name='90/904331/Salt_bridge_859_140/3'>E859 and R140</scene>, <scene name='90/904331/Salt_bridge_974_136/4'>E974 and R136</scene>, and <scene name='90/904331/Salt_bridge_978_123_133/3'>E978 with both R123 and R133</scene>. These strong ionic interactions also induce the conformational changes in the extracellular domain that induce the signaling pathway. <ref name="Reshetnyak" /> |
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
=== Dimerization of ALK === | === Dimerization of ALK === | ||
Revision as of 03:39, 21 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
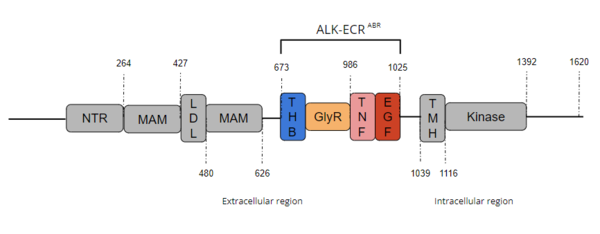
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997 Jan 30;14(4):439-49. doi: 10.1038/sj.onc.1200849. PMID:9053841 doi:http://dx.doi.org/10.1038/sj.onc.1200849
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 3.0 3.1 3.2 Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, Ciardiello F, Morgillo F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol Cancer. 2018 Feb 19;17(1):30. doi: 10.1186/s12943-018-0776-2. PMID:29455642 doi:http://dx.doi.org/10.1186/s12943-018-0776-2
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ 6.0 6.1 6.2 6.3 Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 7.0 7.1 7.2 Chen S, Wang B, Fu X, Liang Y, Chai X, Ye Z, Li R, He Y, Kong G, Lian J, Li X, Chen T, Zhang X, Qiu X, Tang X, Zhou K, Lin B, Zeng J. ALKAL1 gene silencing prevents colorectal cancer progression via suppressing Sonic Hedgehog (SHH) signaling pathway. J Cancer. 2021 Jan 1;12(1):150-162. doi: 10.7150/jca.46447. eCollection 2021. PMID:33391411 doi:http://dx.doi.org/10.7150/jca.46447
- ↑ Reshetnyak AV, Murray PB, Shi X, Mo ES, Mohanty J, Tome F, Bai H, Gunel M, Lax I, Schlessinger J. Augmentor alpha and beta (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):15862-7. doi:, 10.1073/pnas.1520099112. Epub 2015 Nov 16. PMID:26630010 doi:http://dx.doi.org/10.1073/pnas.1520099112
- ↑ De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ 10.0 10.1 10.2 10.3 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 11.0 11.1 Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, Chow AK, Weiser DA, Belcastro LT, Winter C, Bresler SC, Vigny M, Mazot P, Asgharzadeh S, Seeger RC, Zhao H, Guo R, Christensen JG, Orange JS, Pawel BR, Lemmon MA, Mosse YP. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012 Nov 15;31(46):4859-67. doi: 10.1038/onc.2011.647. Epub 2012 Jan, 23. PMID:22266870 doi:http://dx.doi.org/10.1038/onc.2011.647