Thymidylate synthase
From Proteopedia
| Line 18: | Line 18: | ||
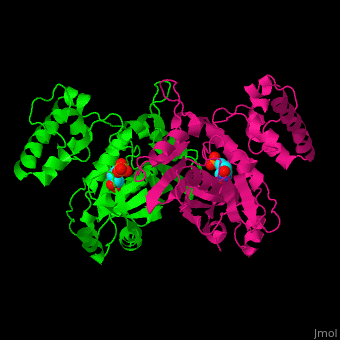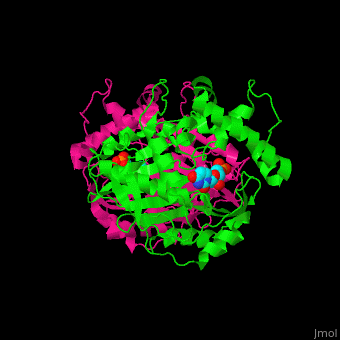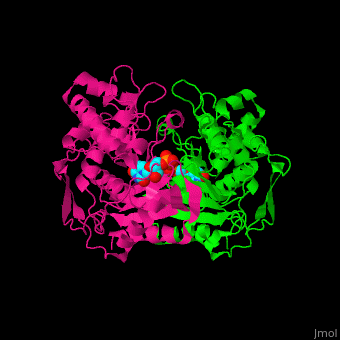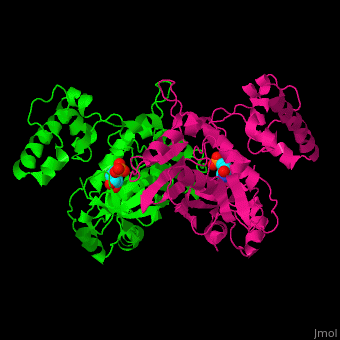
<StructureSection load='' size='350' side='right' caption='Thymidylate synthase complex with dUMP (PDB entry [[1tsv]])' scene='49/493689/Cv/1'> | <StructureSection load='' size='350' side='right' caption='Thymidylate synthase complex with dUMP (PDB entry [[1tsv]])' scene='49/493689/Cv/1'> | ||
==Structure and ligand binding== | ==Structure and ligand binding== | ||
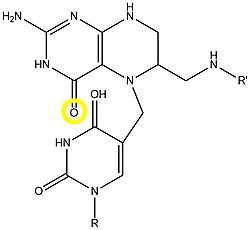
| - | TS forms a homodimer consisting of two domains (reload <scene name='49/493689/Cv/1'>initial scene</scene>). Their <scene name='49/493689/Dump_binding/2'>active sites</scene> can each bind the substrate dUMP<ref>PMID:9053905</ref>. | + | TS forms a homodimer consisting of two domains (reload <scene name='49/493689/Cv/1'>initial scene</scene>). Their <scene name='49/493689/Dump_binding/2'>active sites</scene> can each bind the substrate dUMP<ref>PMID:9053905</ref>. The sketch below shows some of the ionic and hydrogen bonding interactions between the protein and the substrate, most of which are also shown in the 3D scene. You can measure distances and angles after turning off the spinning. |
| - | + | [[Image:DUMP binding.PNG|300px]] | |
| + | |||
| + | Maley et al. show that E.coli TS uses a half-the-sites mechanism when catalyzing its reaction<ref>DOI:10.1021/bi00005a001</ref>. One of the two active sites catalyzes the reaction while the other remains inactive. The way the two domains communicate with each other to coordinate this is still unknown. | ||
| + | |||
| + | To distinguish between path A and path B in the mechanistic hypotheses depicted above, Kholodar et al co-crystallized the enzyme with an analog of the <scene name='49/493689/Ts_7jxf_percentb/4'>bisubstrate intermediate</scene>. | ||
| + | [[Image:IntB.jpg|250px]] | ||
| + | |||
| + | The active site cysteine can covalently bind to and unbind from the appropriate carbon on the dUMP-ring, as seen here in the analog of intermediate conformations. The swtich of the carboxylic oxygen (circled in yellow) of the folate to nitrogen (<jmol> | ||
| + | <jmolLink> | ||
| + | <script> | ||
| + | define current selected; | ||
| + | select [VNM]301:B.NA4%B #9178; | ||
| + | selectionHalos on; | ||
| + | delay 0.5; | ||
| + | selectionHalos off; | ||
| + | select current; | ||
| + | define current selected; | ||
| + | select [VNM]301:B.NA4%A #9177; | ||
| + | selectionHalos on; | ||
| + | delay 0.5; | ||
| + | selectionHalos off; | ||
| + | select current; | ||
| + | </script> | ||
| + | <text>☼</text> | ||
| + | </jmolLink> | ||
| + | </jmol>) in the intermediate prevents the reaction from progressing to its products. | ||
| + | |||
| + | In the structure, the active site csteine has two conformations. One conformation shows the active site cysteine 4.21 Angstroms away from the C6 carbon of the dMP ring and the other 3.84 Angstroms away from the C6 carbon of the dMP ring. | ||
<jmol> | <jmol> | ||
<jmolRadioGroup> | <jmolRadioGroup> | ||
| Line 41: | Line 68: | ||
</jmol> | </jmol> | ||
| - | The active site cysteine can covalently bind to and unbind from the appropriate carbon on the dUMP-ring, as seen here in the analog of intermediate conformations. The conversion of the carboxylic oxygen (circled in yellow) of the folate to nitrogen (<jmol> | ||
| - | <jmolLink> | ||
| - | <script> | ||
| - | define current selected; | ||
| - | select [VNM]301:B.NA4%B #9178; | ||
| - | selectionHalos on; | ||
| - | delay 0.5; | ||
| - | selectionHalos off; | ||
| - | select current; | ||
| - | define current selected; | ||
| - | select [VNM]301:B.NA4%A #9177; | ||
| - | selectionHalos on; | ||
| - | delay 0.5; | ||
| - | selectionHalos off; | ||
| - | select current; | ||
| - | </script> | ||
| - | <text>☼</text> | ||
| - | </jmolLink> | ||
| - | </jmol>) in the intermediate prevents the reaction from progressing to its products. | ||
| - | + | ||
==Inhibition== | ==Inhibition== | ||
Revision as of 12:51, 21 April 2022
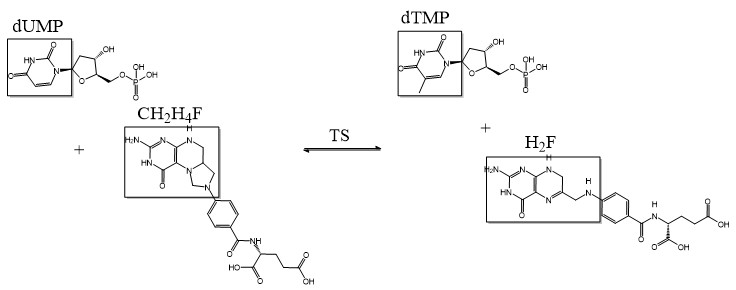
Thymidylate Synthase (TS) is an important enzyme in one-carbon metabolism. TS catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. [1]
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH2H4F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H2F)
Contents |
Function
Thymidylate synthase (TS) catalyzes the methylation of dUMP to dTMP using 5,10-methylenetetrahydrofolate as a cofactor. TS is essential for DNA replication and repair[2]. In protozoa, dihydrofolate reductase (DHFR) and TS are expressed as a bifunctional monomeric enzyme (DHFR-TS) with the DHFR entity at the N terminal. DHFR and TS catalyze consecutive reactions in the dTMP biosynthesis. There are two different types of TS – ThyA and ThyX. The types differ in their activity and structure. The TS ThyX are flavin-dependent enzymes.
Relevance
One of the nucleobase of DNA, cytosine, spontaneously deaminates to form uracil, changing the encoded message. Different from RNA, DNA does not contain uracil but instead contains the methylated form thymine, avoiding missense mutations. The DNA repair protein Uracil DNA glycosylase will recognize uracil as damaged DNA, removing it and re-establishing the original message[3]. Because thymine contains an additional methyl group, it is distinguishable from uracil, and is not removed through DNA repair. Thymidylate synthase helps to turn the abundant uridine (containing the nucleobase uracil) into deoxythymidine (containing the nucleobase thymine) in preparation for DNA synthesis prior to cell division. Thus, thymidylate synthase plays a role in making DNA a more reliable long-term storage of genetic information compared to RNA.
TS inhibition at its folate-binding site is used in anticancer therapeutic drugs. DHFR-TS inhibitors are potential drug targets against parasite-transferred diseases. TS exhibits oncogene-like activity.[4]
Mechanism
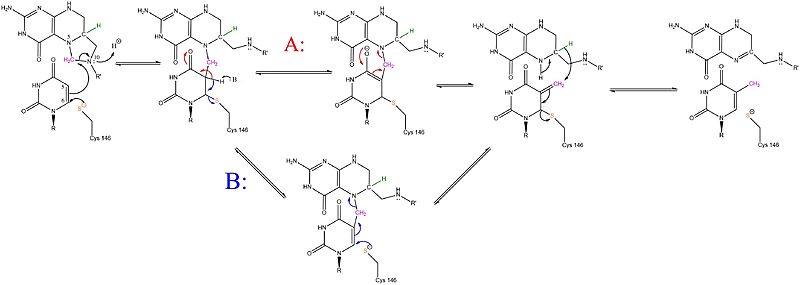
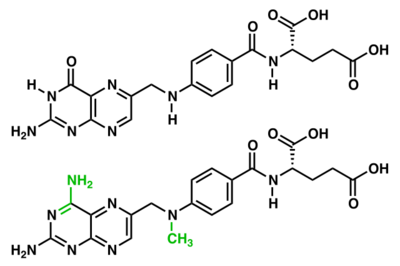
There are two potential pathways that the mechanism for TS could operate by, however it is unclear which pathway the mechanism follows. In both pathways, the substrates form a covalent ternary complex with the enzyme. This complex facilitates the transfer of the methyl group from CH2H4F to dUMP and is followed by a hydride transfer from the folate substrate to the dMP substrate. During the A pathway (highlighted in red), the intermediate complex remains covalently bound to the enzyme. The B pathway (highlighted in blue) involves the decoupling of the intermediate from the active site cysteine.
| |||||||||||
3D structures of thymidylate synthase
Thymidylate synthase 3D structures
References
- ↑ Kholodar SA, Finer-Moore JS, Swiderek K, Arafet K, Moliner V, Stroud RM, Kohen A. Caught in Action: X-ray Structure of Thymidylate Synthase with Noncovalent Intermediate Analog. Biochemistry. 2021 Apr 8. doi: 10.1021/acs.biochem.1c00063. PMID:33829766 doi:http://dx.doi.org/10.1021/acs.biochem.1c00063
- ↑ Kaneda S, Nalbantoglu J, Takeishi K, Shimizu K, Gotoh O, Seno T, Ayusawa D. Structural and functional analysis of the human thymidylate synthase gene. J Biol Chem. 1990 Nov 25;265(33):20277-84. PMID:2243092
- ↑ Krokan HE, Drablos F, Slupphaug G. Uracil in DNA--occurrence, consequences and repair. Oncogene. 2002 Dec 16;21(58):8935-48. doi: 10.1038/sj.onc.1205996. PMID:12483510 doi:http://dx.doi.org/10.1038/sj.onc.1205996
- ↑ Rahman L, Voeller D, Rahman M, Lipkowitz S, Allegra C, Barrett JC, Kaye FJ, Zajac-Kaye M. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004 Apr;5(4):341-51. PMID:15093541
- ↑ Finer-Moore JS, Fauman EB, Morse RJ, Santi DV, Stroud RM. Contribution of a salt bridge to binding affinity and dUMP orientation to catalytic rate: mutation of a substrate-binding arginine in thymidylate synthase. Protein Eng. 1996 Jan;9(1):69-75. PMID:9053905
- ↑ Maley F, Pedersen-Lane J, Changchien L. Complete restoration of activity to inactive mutants of Escherichia coli thymidylate synthase: evidence that E. coli thymidylate synthase is a half-the-sites activity enzyme. Biochemistry. 1995 Feb 7;34(5):1469-74. doi: 10.1021/bi00005a001. PMID:7849005 doi:http://dx.doi.org/10.1021/bi00005a001
- ↑ Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12(19):2241-58. doi: 10.2174/0929867054864868. PMID:16178783 doi:http://dx.doi.org/10.2174/0929867054864868
Proteopedia Page Contributors and Editors (what is this?)
Michael O'Shaughnessy, Karsten Theis, Michal Harel, Alexander Berchansky, Shaylie Albright, Anna Postnikova, Kia Yang, Joel L. Sussman