Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='7N00' size='350' side='right' caption=' Structure of Anaplastic Lymphoma Kinase [https://www.rcsb.org/structure/7N00 7N00]' scene='90/904331/Alk_full/1'> | <StructureSection load='7N00' size='350' side='right' caption=' Structure of Anaplastic Lymphoma Kinase [https://www.rcsb.org/structure/7N00 7N00]' scene='90/904331/Alk_full/1'> | ||
== Background == | == Background == | ||
| - | Anaplastic Lymphoma Kinase (ALK) is a [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] receptor and a member of the family of [https://proteopedia.org/wiki/index.php/Receptor_tyrosine_kinases Receptor Tyrosine Kinases (RTKs)]<ref name="Iwahara">PMID:9053841</ref>. RTKs are a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway.<ref name="Huang" /> ALK was identified as a novel tyrosine phosphoprotein in 1994 in an analysis of [https://lymphoma.org/aboutlymphoma/nhl/alcl/ Anaplastic Large-Cell Lymphoma], the protein's namesake.<ref name ="Huang" /> A full analysis and characterization of ALK was completed in 1997, properly identifying it as an RTK, and linking it closely to [https://en.wikipedia.org/wiki/Leukocyte_receptor_tyrosine_kinase Leukocyte Tyrosine Kinase] (LTK).<ref name ="Huang" /> ALK's normal activity as a receptor tyrosine kinase is to transfer a gamma-phosphate group from adenosine triphosphate (ATP) to a tyrosine residue on its substrate.<ref name ="Huang" /> ALK is one of more than 50 RTKs encoded within the human genome, <ref name ="Huang" /> and its tyrosine kinase activity seems to be especially important in the developing nervous system. <ref name ="Huang" /> The ALK extracellular domain is becoming well characterized, as it is the primary binding site of ALK ligands that end up triggering the kinase activity, as ALK autophosphorylates itself.<ref name="Reshetnyak" /> There are four well-characterized domains on the ALK extracellular domain that are involved in binding the two main ligands of ALK | + | Anaplastic Lymphoma Kinase (ALK) is a [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] receptor and a member of the family of [https://proteopedia.org/wiki/index.php/Receptor_tyrosine_kinases Receptor Tyrosine Kinases (RTKs)]<ref name="Iwahara">PMID:9053841</ref>. RTKs are a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway.<ref name="Huang" /> ALK was identified as a novel tyrosine phosphoprotein in 1994 in an analysis of [https://lymphoma.org/aboutlymphoma/nhl/alcl/ Anaplastic Large-Cell Lymphoma], the protein's namesake.<ref name ="Huang" /> A full analysis and characterization of ALK was completed in 1997, properly identifying it as an RTK, and linking it closely to [https://en.wikipedia.org/wiki/Leukocyte_receptor_tyrosine_kinase Leukocyte Tyrosine Kinase] (LTK).<ref name ="Huang" /> ALK's normal activity as a receptor tyrosine kinase is to transfer a gamma-phosphate group from adenosine triphosphate (ATP) to a tyrosine residue on its substrate.<ref name ="Huang" /> ALK is one of more than 50 RTKs encoded within the human genome, <ref name ="Huang" /> and its tyrosine kinase activity seems to be especially important in the developing nervous system. <ref name ="Huang" /> The ALK extracellular domain is becoming well characterized, as it is the primary binding site of ALK ligands that end up triggering the kinase activity, as ALK autophosphorylates itself.<ref name="Reshetnyak" /> There are four well-characterized domains on the ALK extracellular domain that are involved in binding the two main ligands of ALK.<ref name="Reshetnyak" /> ALK is most commonly associated with oncogenesis, as various factors, including overstimulation, lead to extreme cell proliferation.<ref name="Della Corte" /> It is primarily found in the fetal and infant developing nervous system, however when associated with cancer it can be found in systems other than the nervous system.<ref name="Carpenter" /> Examples of these include colon and prostate cancer, where ALK is not normally expressed.<ref name="Chen" /> This particular protein is interesting in part due to its unique structure, of that it shares the most similarity with LTKs and its association with devastating cancers.<ref name="DeMunck" /> |
== Structure == | == Structure == | ||
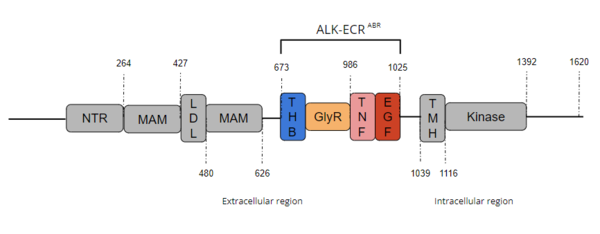
ALK is a close homolog of LTK, and together these two homologs constitute a subgroup within the superfamily of [https://proteopedia.org/wiki/index.php/Insulin_receptor insulin receptors]<ref name="Della Corte" />. ALK is composed of three primary regions: the extracellular region, the transmembrane region, and the intracellular region.<ref name="Reshetnyak" /> [[Image:Full ALK Structure Graphic.PNG|600 px|right|thumb|Figure 1. Overview of Anaplastic Lymphoma Kinase Structure with domains where known structures are color coordinated and other domains are grayed out. The abbreviations are as follows: NTR-N-terminal Region, MAM-Meprin-A-5 protein-receptor protein tyrosine phosphatase μ, LDL-low-density lipoprotein receptor class A, THB-three helix bundle-like, GlyR-poly-glycine region, TNF-tumor necrosis factor-like domain, EGF-epidermal growth factor-like, TMH-transmembrane helix]] The extracellular region of ALK contains 8 total domains within 2 fragments.<ref name="Reshetnyak" /> A Three Helix Bundle-like domain (THB-like), a Poly-Glycine domain (GlyR), a Tumor Necrosis Factor-like domain (TNF-like), and an Epidermal Growth Factor-like domain (EGF-like) make up the ligand binding fragment while an N-terminal domain, two [https://en.wikipedia.org/wiki/Meprin_A meprin–A-5] protein–receptor protein tyrosine phosphatase μ (MAM) domains and a [https://en.wikipedia.org/wiki/Low-density_lipoprotein low-density lipoprotein] receptor class A (LDL) domain sandwiched between the two MAM domains make up the second fragment.<ref name="Reshetnyak" /> All four domains of the ligand binding fragment of the extracellular region contribute to ligand-binding <ref name ="Huang" />. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear, but the MAM region has been hypothesized to play a role in cell-cell signaling<ref name="Palmer">PMID:19459784</ref>. The [https://en.wikipedia.org/wiki/Transmembrane_domain transmembrane helical region] (TMH) bridges the gap between the intracellular and extracellular regions.<ref name="Reshetnyak" /> The intracellular tyrosine kinase region features the Kinase domain and the C-terminal end (Figure 1). <ref name="Reshetnyak" /> | ALK is a close homolog of LTK, and together these two homologs constitute a subgroup within the superfamily of [https://proteopedia.org/wiki/index.php/Insulin_receptor insulin receptors]<ref name="Della Corte" />. ALK is composed of three primary regions: the extracellular region, the transmembrane region, and the intracellular region.<ref name="Reshetnyak" /> [[Image:Full ALK Structure Graphic.PNG|600 px|right|thumb|Figure 1. Overview of Anaplastic Lymphoma Kinase Structure with domains where known structures are color coordinated and other domains are grayed out. The abbreviations are as follows: NTR-N-terminal Region, MAM-Meprin-A-5 protein-receptor protein tyrosine phosphatase μ, LDL-low-density lipoprotein receptor class A, THB-three helix bundle-like, GlyR-poly-glycine region, TNF-tumor necrosis factor-like domain, EGF-epidermal growth factor-like, TMH-transmembrane helix]] The extracellular region of ALK contains 8 total domains within 2 fragments.<ref name="Reshetnyak" /> A Three Helix Bundle-like domain (THB-like), a Poly-Glycine domain (GlyR), a Tumor Necrosis Factor-like domain (TNF-like), and an Epidermal Growth Factor-like domain (EGF-like) make up the ligand binding fragment while an N-terminal domain, two [https://en.wikipedia.org/wiki/Meprin_A meprin–A-5] protein–receptor protein tyrosine phosphatase μ (MAM) domains and a [https://en.wikipedia.org/wiki/Low-density_lipoprotein low-density lipoprotein] receptor class A (LDL) domain sandwiched between the two MAM domains make up the second fragment.<ref name="Reshetnyak" /> All four domains of the ligand binding fragment of the extracellular region contribute to ligand-binding <ref name ="Huang" />. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear, but the MAM region has been hypothesized to play a role in cell-cell signaling<ref name="Palmer">PMID:19459784</ref>. The [https://en.wikipedia.org/wiki/Transmembrane_domain transmembrane helical region] (TMH) bridges the gap between the intracellular and extracellular regions.<ref name="Reshetnyak" /> The intracellular tyrosine kinase region features the Kinase domain and the C-terminal end (Figure 1). <ref name="Reshetnyak" /> | ||
Current revision
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997 Jan 30;14(4):439-49. doi: 10.1038/sj.onc.1200849. PMID:9053841 doi:http://dx.doi.org/10.1038/sj.onc.1200849
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 3.25 3.26 3.27 3.28 3.29 3.30 3.31 3.32 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ 4.0 4.1 4.2 4.3 Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, Ciardiello F, Morgillo F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol Cancer. 2018 Feb 19;17(1):30. doi: 10.1186/s12943-018-0776-2. PMID:29455642 doi:http://dx.doi.org/10.1186/s12943-018-0776-2
- ↑ 5.0 5.1 5.2 Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, Chow AK, Weiser DA, Belcastro LT, Winter C, Bresler SC, Vigny M, Mazot P, Asgharzadeh S, Seeger RC, Zhao H, Guo R, Christensen JG, Orange JS, Pawel BR, Lemmon MA, Mosse YP. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012 Nov 15;31(46):4859-67. doi: 10.1038/onc.2011.647. Epub 2012 Jan, 23. PMID:22266870 doi:http://dx.doi.org/10.1038/onc.2011.647
- ↑ 6.0 6.1 6.2 6.3 Chen S, Wang B, Fu X, Liang Y, Chai X, Ye Z, Li R, He Y, Kong G, Lian J, Li X, Chen T, Zhang X, Qiu X, Tang X, Zhou K, Lin B, Zeng J. ALKAL1 gene silencing prevents colorectal cancer progression via suppressing Sonic Hedgehog (SHH) signaling pathway. J Cancer. 2021 Jan 1;12(1):150-162. doi: 10.7150/jca.46447. eCollection 2021. PMID:33391411 doi:http://dx.doi.org/10.7150/jca.46447
- ↑ 7.0 7.1 De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ 9.0 9.1 Reshetnyak AV, Murray PB, Shi X, Mo ES, Mohanty J, Tome F, Bai H, Gunel M, Lax I, Schlessinger J. Augmentor alpha and beta (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):15862-7. doi:, 10.1073/pnas.1520099112. Epub 2015 Nov 16. PMID:26630010 doi:http://dx.doi.org/10.1073/pnas.1520099112
- ↑ 10.0 10.1 10.2 10.3 Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 11.0 11.1 11.2 11.3 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7