User:David Gucklhorn/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structure == | == Structure == | ||
| - | ERBB receptors contain an extracellular domain (ECD), a transmembrane domain (TMD), an intracellular region that consists of a juxtamembrane domain (JMD), a kinase domain (KD) and a carboxy terminal tail domain (CTD) | + | ERBB receptors contain an extracellular domain (ECD), a transmembrane domain (TMD), an intracellular region that consists of a juxtamembrane domain (JMD), a kinase domain (KD) and a carboxy terminal tail domain (CTD) <ref>DOI 10.1146/annurev-biochem-060614-034402</ref>. The ECD is comprised of four subdomains (I-IV). In the absence of ligand, the ECD adopts an auto-inhibited tethered (closed) conformation that involves domain II and IV. Upon ligand binding between domains I and III, the dimerization arm in domain II is untethered, leading to receptor homo or heterodimerization, allosteric kinase activation, CTD phosphorylation and downstream signaling <ref>DOI 10.1146/annurev-biochem-060614-034402</ref><ref>DOI 10.1016/j.ccell.2018.09.010</ref>. |
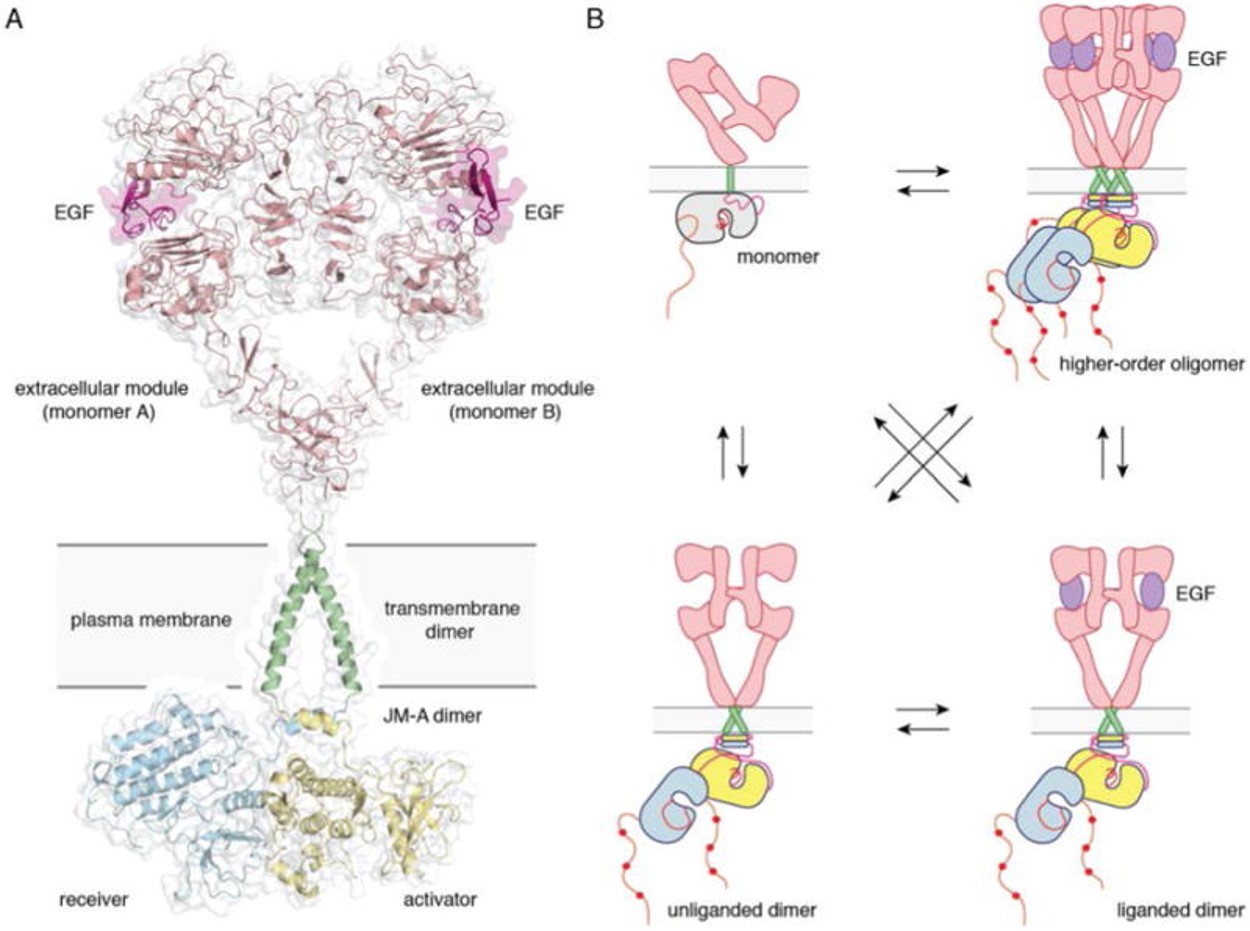
[[Image:Dg_sb_Figure1.jpg|frame|center|Full-length EGFR and its oligomerization states | [[Image:Dg_sb_Figure1.jpg|frame|center|Full-length EGFR and its oligomerization states | ||
| - | A) A proposed composite model of full-length EGFR based on the structures of individual modules (PDB ID 3NJP for the extracellular module, PDB ID 2M20 for the transmembrane - JM-A helices, and PDB ID 2GS6 for the kinase domains). B) Schematics for possible oligomerization states of EGFR in cells. | + | A) A proposed composite model of full-length EGFR based on the structures of individual modules (PDB ID 3NJP for the extracellular module, PDB ID 2M20 for the transmembrane - JM-A helices, and PDB ID 2GS6 for the kinase domains). B) Schematics for possible oligomerization states of EGFR in cells. <ref>DOI 10.1146/annurev-biochem-060614-034402</ref> |
]] | ]] | ||
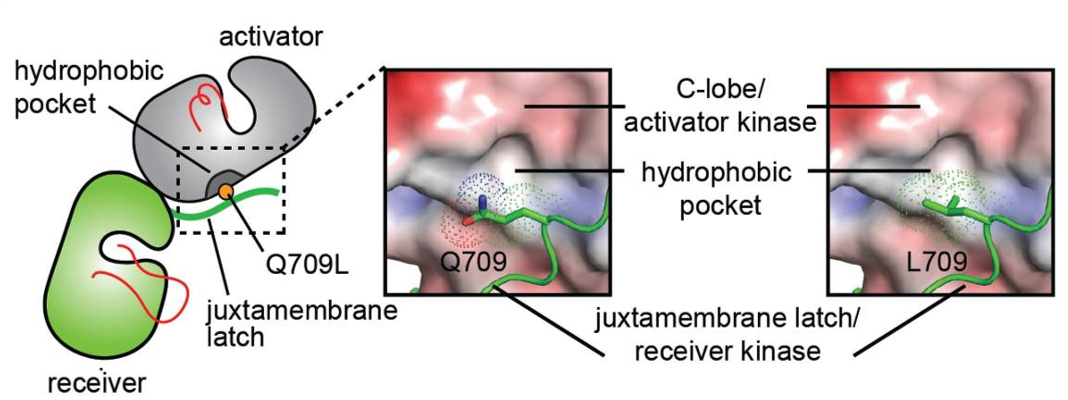
HER2 is an atypical member of the ERBB family, as its ECD adopts an untethered conformation constitutively (Roskoski, 2014). Unlike the other ERBB family members, HER2 does not have a ligand. HER2 preferentially heterodimerizes with ligand bound untethered (open) HER3 or EGFR to initiate cellular signaling, although HER2 homodimers capable of signaling have been reported in HER2 overexpressing cells (Brennan et al., 2000; Roskoski, 2014). <ref>DOI 10.1016/j.ccell.2018.09.010</ref> | HER2 is an atypical member of the ERBB family, as its ECD adopts an untethered conformation constitutively (Roskoski, 2014). Unlike the other ERBB family members, HER2 does not have a ligand. HER2 preferentially heterodimerizes with ligand bound untethered (open) HER3 or EGFR to initiate cellular signaling, although HER2 homodimers capable of signaling have been reported in HER2 overexpressing cells (Brennan et al., 2000; Roskoski, 2014). <ref>DOI 10.1016/j.ccell.2018.09.010</ref> | ||
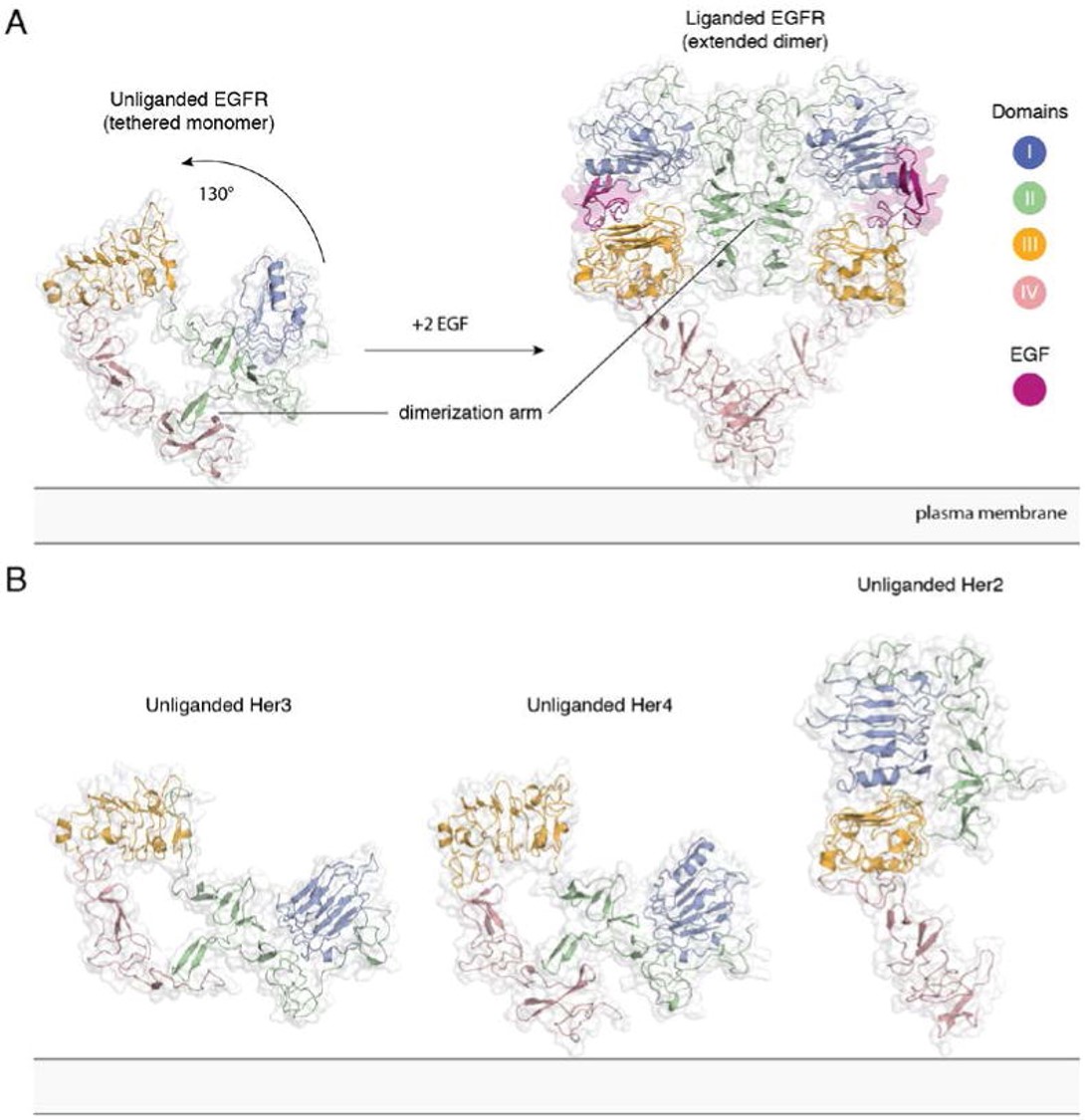
[[Image:Dg_sb_Figure2.jpg|frame|center|Extracellular module structures for the EGFR family members | [[Image:Dg_sb_Figure2.jpg|frame|center|Extracellular module structures for the EGFR family members | ||
| - | A) The conformational change induced by ligand binding. The tethered conformation of EGFR (left, PDB ID 1NQL, EGF bound at low pH was removed for clarity) rearranges to the extended conformation of EGFR (right, PDB ID 3NJP) upon ligand binding. B) Unliganded Her3 (PDB ID 1M6B) and Her4 (PDB ID 2AHX) can adopt a tethered conformation similar to EGFR, while Her2 (PDB ID 1N8H) is in an extended conformation, even in the absence of ligand.]] | + | A) The conformational change induced by ligand binding. The tethered conformation of EGFR (left, PDB ID 1NQL, EGF bound at low pH was removed for clarity) rearranges to the extended conformation of EGFR (right, PDB ID 3NJP) upon ligand binding. B) Unliganded Her3 (PDB ID 1M6B) and Her4 (PDB ID 2AHX) can adopt a tethered conformation similar to EGFR, while Her2 (PDB ID 1N8H) is in an extended conformation, even in the absence of ligand.<ref>DOI 10.1146/annurev-biochem-060614-034402</ref>]] |
Revision as of 20:47, 27 April 2022
ErbB2
| |||||||||||
References
- ↑ Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997 Apr 1;16(7):1647-55. doi: 10.1093/emboj/16.7.1647. PMID:9130710 doi:http://dx.doi.org/10.1093/emboj/16.7.1647
- ↑ Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990 Jun 29;61(7):1339-47. doi: 10.1016/0092-8674(90)90697-d. PMID:1973074 doi:http://dx.doi.org/10.1016/0092-8674(90)90697-d
- ↑ Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006 Jul;7(7):505-16. doi: 10.1038/nrm1962. PMID:16829981 doi:http://dx.doi.org/10.1038/nrm1962
- ↑ Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402., Epub 2015 Jan 26. PMID:25621509 doi:http://dx.doi.org/10.1146/annurev-biochem-060614-034402
- ↑ Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402., Epub 2015 Jan 26. PMID:25621509 doi:http://dx.doi.org/10.1146/annurev-biochem-060614-034402
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402., Epub 2015 Jan 26. PMID:25621509 doi:http://dx.doi.org/10.1146/annurev-biochem-060614-034402
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402., Epub 2015 Jan 26. PMID:25621509 doi:http://dx.doi.org/10.1146/annurev-biochem-060614-034402
- ↑ Slamon DJ, Clark GM. Amplification of c-erbB-2 and aggressive human breast tumors? Science. 1988 Jun 24;240(4860):1795-8. doi: 10.1126/science.3289120. PMID:3289120 doi:http://dx.doi.org/10.1126/science.3289120
- ↑ Venter DJ, Tuzi NL, Kumar S, Gullick WJ. Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet. 1987 Jul 11;2(8550):69-72. doi: 10.1016/s0140-6736(87)92736-x. PMID:2885574 doi:http://dx.doi.org/10.1016/s0140-6736(87)92736-x
- ↑ Fleishman SJ, Schlessinger J, Ben-Tal N. A putative molecular-activation switch in the transmembrane domain of erbB2. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):15937-40. doi:, 10.1073/pnas.252640799. Epub 2002 Dec 2. PMID:12461170 doi:http://dx.doi.org/10.1073/pnas.252640799
- ↑ Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, Zhang X, Lee SH, Cho J, Ambrogio L, Liao R, Imielinski M, Banerji S, Berger AH, Lawrence MS, Zhang J, Pho NH, Walker SR, Winckler W, Getz G, Frank D, Hahn WC, Eck MJ, Mani DR, Jaffe JD, Carr SA, Wong KK, Meyerson M. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14476-81. doi:, 10.1073/pnas.1203201109. Epub 2012 Aug 20. PMID:22908275 doi:http://dx.doi.org/10.1073/pnas.1203201109
- ↑ Zabransky DJ, Yankaskas CL, Cochran RL, Wong HY, Croessmann S, Chu D, Kavuri SM, Red Brewer M, Rosen DM, Dalton WB, Cimino-Mathews A, Cravero K, Button B, Kyker-Snowman K, Cidado J, Erlanger B, Parsons HA, Manto KM, Bose R, Lauring J, Arteaga CL, Konstantopoulos K, Park BH. HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc Natl Acad Sci U S A. 2015 Nov 10;112(45):E6205-14. doi:, 10.1073/pnas.1516853112. Epub 2015 Oct 27. PMID:26508629 doi:http://dx.doi.org/10.1073/pnas.1516853112
- ↑ Ross JS, Gay LM, Wang K, Ali SM, Chumsri S, Elvin JA, Bose R, Vergilio JA, Suh J, Yelensky R, Lipson D, Chmielecki J, Waintraub S, Leyland-Jones B, Miller VA, Stephens PJ. Nonamplification ERBB2 genomic alterations in 5605 cases of recurrent and metastatic breast cancer: An emerging opportunity for anti-HER2 targeted therapies. Cancer. 2016 Sep 1;122(17):2654-62. doi: 10.1002/cncr.30102. Epub 2016 Jun 10. PMID:27284958 doi:http://dx.doi.org/10.1002/cncr.30102
- ↑ Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, Ma CX, Ding L, Mardis ER, Ellis MJ. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013 Feb;3(2):224-37. doi: 10.1158/2159-8290.CD-12-0349. Epub 2012, Dec 7. PMID:23220880 doi:http://dx.doi.org/10.1158/2159-8290.CD-12-0349
- ↑ Yamamoto H, Higasa K, Sakaguchi M, Shien K, Soh J, Ichimura K, Furukawa M, Hashida S, Tsukuda K, Takigawa N, Matsuo K, Kiura K, Miyoshi S, Matsuda F, Toyooka S. Novel germline mutation in the transmembrane domain of HER2 in familial lung adenocarcinomas. J Natl Cancer Inst. 2014 Jan;106(1):djt338. doi: 10.1093/jnci/djt338. Epub 2013, Dec 7. PMID:24317180 doi:http://dx.doi.org/10.1093/jnci/djt338
- ↑ Kavuri SM, Jain N, Galimi F, Cottino F, Leto SM, Migliardi G, Searleman AC, Shen W, Monsey J, Trusolino L, Jacobs SA, Bertotti A, Bose R. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015 Aug;5(8):832-41. doi: 10.1158/2159-8290.CD-14-1211. PMID:26243863 doi:http://dx.doi.org/10.1158/2159-8290.CD-14-1211
- ↑ Ou SI, Schrock AB, Bocharov EV, Klempner SJ, Haddad CK, Steinecker G, Johnson M, Gitlitz BJ, Chung J, Campregher PV, Ross JS, Stephens PJ, Miller VA, Suh JH, Ali SM, Velcheti V. HER2 Transmembrane Domain (TMD) Mutations (V659/G660) That Stabilize Homo- and Heterodimerization Are Rare Oncogenic Drivers in Lung Adenocarcinoma That Respond to Afatinib. J Thorac Oncol. 2017 Mar;12(3):446-457. doi: 10.1016/j.jtho.2016.11.2224. Epub, 2016 Nov 27. PMID:27903463 doi:http://dx.doi.org/10.1016/j.jtho.2016.11.2224
- ↑ Chang MT, Bhattarai TS, Schram AM, Bielski CM, Donoghue MTA, Jonsson P, Chakravarty D, Phillips S, Kandoth C, Penson A, Gorelick A, Shamu T, Patel S, Harris C, Gao J, Sumer SO, Kundra R, Razavi P, Li BT, Reales DN, Socci ND, Jayakumaran G, Zehir A, Benayed R, Arcila ME, Chandarlapaty S, Ladanyi M, Schultz N, Baselga J, Berger MF, Rosen N, Solit DB, Hyman DM, Taylor BS. Accelerating Discovery of Functional Mutant Alleles in Cancer. Cancer Discov. 2018 Feb;8(2):174-183. doi: 10.1158/2159-8290.CD-17-0321. Epub, 2017 Dec 15. PMID:29247016 doi:http://dx.doi.org/10.1158/2159-8290.CD-17-0321
- ↑ Petrelli F, Tomasello G, Barni S, Lonati V, Passalacqua R, Ghidini M. Clinical and pathological characterization of HER2 mutations in human breast cancer: a systematic review of the literature. Breast Cancer Res Treat. 2017 Nov;166(2):339-349. doi: 10.1007/s10549-017-4419-x., Epub 2017 Jul 31. PMID:28762010 doi:http://dx.doi.org/10.1007/s10549-017-4419-x
- ↑ Cousin S, Khalifa E, Crombe A, Laizet Y, Lucchesi C, Toulmonde M, Le Moulec S, Auzanneau C, Soubeyran I, Italiano A. Targeting ERBB2 mutations in solid tumors: biological and clinical implications. J Hematol Oncol. 2018 Jun 25;11(1):86. doi: 10.1186/s13045-018-0630-4. PMID:29941010 doi:http://dx.doi.org/10.1186/s13045-018-0630-4
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010
- ↑ Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018 Nov 12;34(5):792-806.e5. doi: 10.1016/j.ccell.2018.09.010. Epub, 2018 Oct 25. PMID:30449325 doi:http://dx.doi.org/10.1016/j.ccell.2018.09.010