User:Apolena Zounarová/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
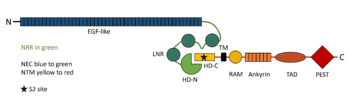
Stable association of the two NOTCH1 chains depends on the heterodimerization domain which consists of two regions. 65 amino acid C-terminal region (HD-C, NTM) remains associated with the transmembrane part of the receptor, whereas 103 aminoacid extracellular N-terminal region (HD-N, NEC) has been separated by furin-type convertase and interacts with the membrane-bound part non-covalently. Adjacent to HD-N are three LIN-12/NOTCH repeats (LNR) which are not necessary for heterodimerization, but rather protect HD-C from metalloprotease cleavage and prevent ligand-independent activation of the signalling pathway. LNR are together with HD-N termed as the negative regulatory region, NRR <ref name="sanchez">DOI 10.1128/MCB.24.21.9265-9273.2004</ref>. | Stable association of the two NOTCH1 chains depends on the heterodimerization domain which consists of two regions. 65 amino acid C-terminal region (HD-C, NTM) remains associated with the transmembrane part of the receptor, whereas 103 aminoacid extracellular N-terminal region (HD-N, NEC) has been separated by furin-type convertase and interacts with the membrane-bound part non-covalently. Adjacent to HD-N are three LIN-12/NOTCH repeats (LNR) which are not necessary for heterodimerization, but rather protect HD-C from metalloprotease cleavage and prevent ligand-independent activation of the signalling pathway. LNR are together with HD-N termed as the negative regulatory region, NRR <ref name="sanchez">DOI 10.1128/MCB.24.21.9265-9273.2004</ref>. | ||
NOTCH mutants with deleted extracellular domain are constitutively active and are cleaved at the S3 site in a constitutive manner. Also, their activity is equivalent to NOTCH mutants containing the intracellular part only. This observation supports the idea that the conformation of the receptor is important for the changes in accessibility of S3 site. Ligand binding can change the conformation and permits cleavage of NOTCH by γ-secretase either directly or indirectly. On the contrary, constructs bearing also LNR are neither constitutively active nor constitutively cleaved <ref name="kopan">DOI 10.1073/pnas.93.4.1683</ref>. | NOTCH mutants with deleted extracellular domain are constitutively active and are cleaved at the S3 site in a constitutive manner. Also, their activity is equivalent to NOTCH mutants containing the intracellular part only. This observation supports the idea that the conformation of the receptor is important for the changes in accessibility of S3 site. Ligand binding can change the conformation and permits cleavage of NOTCH by γ-secretase either directly or indirectly. On the contrary, constructs bearing also LNR are neither constitutively active nor constitutively cleaved <ref name="kopan">DOI 10.1073/pnas.93.4.1683</ref>. | ||
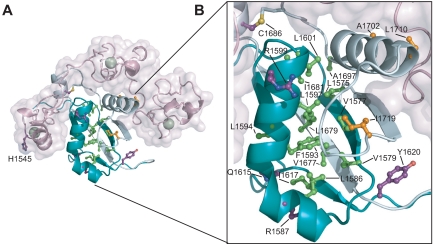
| + | [[Image:HD dom mut.jpg|433px|left|thumb|T-ALL tumor associated mutations in Notch1 NRR. (A) Structural representation highlighting T-ALL mutations. Side chains of residues mutated in T-ALL patients are shown in ball-and-stick form. Residues are colored according to mutation site: core (green), interface (orange), or partially exposed (purple). (B) Close-up view of mutations in the HD domain.]] | ||
== T-cell Acute Lymphoblastic Leukaemia == | == T-cell Acute Lymphoblastic Leukaemia == | ||
Revision as of 13:03, 28 April 2022
NOTCH1 Heterodimerization Domain in T-cell Acute Lymphoblastic Leukaemia
| |||||||||||
References
- ↑ Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587-613. doi:, 10.1146/annurev.pathmechdis.3.121806.154300. PMID:18039126 doi:http://dx.doi.org/10.1146/annurev.pathmechdis.3.121806.154300
- ↑ 2.0 2.1 Gordon WR, Vardar-Ulu D, L'Heureux S, Ashworth T, Malecki MJ, Sanchez-Irizarry C, McArthur DG, Histen G, Mitchell JL, Aster JC, Blacklow SC. Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One. 2009 Aug 24;4(8):e6613. PMID:19701457 doi:10.1371/journal.pone.0006613
- ↑ 3.0 3.1 3.2 . UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021 Jan 8;49(D1):D480-D489. doi: 10.1093/nar/gkaa1100. PMID:33237286 doi:http://dx.doi.org/10.1093/nar/gkaa1100
- ↑ 4.0 4.1 4.2 Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000 Feb;5(2):207-16. doi: 10.1016/s1097-2765(00)80417-7. PMID:10882063 doi:http://dx.doi.org/10.1016/s1097-2765(00)80417-7
- ↑ Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000 Feb;5(2):197-206. doi: 10.1016/s1097-2765(00)80416-5. PMID:10882062 doi:http://dx.doi.org/10.1016/s1097-2765(00)80416-5
- ↑ Nichols JT, Miyamoto A, Olsen SL, D'Souza B, Yao C, Weinmaster G. DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol. 2007 Feb 12;176(4):445-58. doi: 10.1083/jcb.200609014. PMID:17296795 doi:http://dx.doi.org/10.1083/jcb.200609014
- ↑ 7.0 7.1 De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999 Apr 8;398(6727):518-22. doi: 10.1038/19083. PMID:10206645 doi:http://dx.doi.org/10.1038/19083
- ↑ Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998 May 28;393(6683):382-6. doi: 10.1038/30756. PMID:9620803 doi:http://dx.doi.org/10.1038/30756
- ↑ Sanchez-Irizarry C, Carpenter AC, Weng AP, Pear WS, Aster JC, Blacklow SC. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol Cell Biol. 2004 Nov;24(21):9265-73. doi: 10.1128/MCB.24.21.9265-9273.2004. PMID:15485896 doi:http://dx.doi.org/10.1128/MCB.24.21.9265-9273.2004
- ↑ Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1683-8. doi: 10.1073/pnas.93.4.1683. PMID:8643690 doi:http://dx.doi.org/10.1073/pnas.93.4.1683
- ↑ Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008 Mar 22;371(9617):1030-43. doi: 10.1016/S0140-6736(08)60457-2. PMID:18358930 doi:http://dx.doi.org/10.1016/S0140-6736(08)60457-2
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Weng AP, Ferrando AA, Lee W, Morris JP 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004 Oct 8;306(5694):269-71. doi: 10.1126/science.1102160. PMID:15472075 doi:http://dx.doi.org/10.1126/science.1102160
- ↑ Sundaram M, Greenwald I. Suppressors of a lin-12 hypomorph define genes that interact with both lin-12 and glp-1 in Caenorhabditis elegans. Genetics. 1993 Nov;135(3):765-83. PMID:8293978
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 Malecki MJ, Sanchez-Irizarry C, Mitchell JL, Histen G, Xu ML, Aster JC, Blacklow SC. Leukemia-associated mutations within the NOTCH1 heterodimerization domain fall into at least two distinct mechanistic classes. Mol Cell Biol. 2006 Jun;26(12):4642-51. doi: 10.1128/MCB.01655-05. PMID:16738328 doi:http://dx.doi.org/10.1128/MCB.01655-05
- ↑ doi: https://dx.doi.org/10.2210/pdb3I08/pdb
- ↑ Sulis ML, Williams O, Palomero T, Tosello V, Pallikuppam S, Real PJ, Barnes K, Zuurbier L, Meijerink JP, Ferrando AA. NOTCH1 extracellular juxtamembrane expansion mutations in T-ALL. Blood. 2008 Aug 1;112(3):733-40. doi: 10.1182/blood-2007-12-130096. Epub 2008 Apr, 14. PMID:18411416 doi:http://dx.doi.org/10.1182/blood-2007-12-130096
- ↑ Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002 Jun;14(6):637-45. doi: 10.1093/intimm/dxf030. PMID:12039915 doi:http://dx.doi.org/10.1093/intimm/dxf030
- ↑ Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004 Aug 16;200(4):469-79. doi: 10.1084/jem.20040394. PMID:15314075 doi:http://dx.doi.org/10.1084/jem.20040394
- ↑ Washburn T, Schweighoffer E, Gridley T, Chang D, Fowlkes BJ, Cado D, Robey E. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997 Mar 21;88(6):833-43. doi: 10.1016/s0092-8674(00)81929-7. PMID:9118226 doi:http://dx.doi.org/10.1016/s0092-8674(00)81929-7
- ↑ Palomero T, Barnes KC, Real PJ, Glade Bender JL, Sulis ML, Murty VV, Colovai AI, Balbin M, Ferrando AA. CUTLL1, a novel human T-cell lymphoma cell line with t(7;9) rearrangement, aberrant NOTCH1 activation and high sensitivity to gamma-secretase inhibitors. Leukemia. 2006 Jul;20(7):1279-87. doi: 10.1038/sj.leu.2404258. Epub 2006 May 11. PMID:16688224 doi:http://dx.doi.org/10.1038/sj.leu.2404258
- ↑ Palomero T, Lim WK, Odom DT, Sulis ML, Real PJ, Margolin A, Barnes KC, O'Neil J, Neuberg D, Weng AP, Aster JC, Sigaux F, Soulier J, Look AT, Young RA, Califano A, Ferrando AA. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006 Nov 28;103(48):18261-6. doi:, 10.1073/pnas.0606108103. Epub 2006 Nov 17. PMID:17114293 doi:http://dx.doi.org/10.1073/pnas.0606108103
- ↑ Cullion K, Draheim KM, Hermance N, Tammam J, Sharma VM, Ware C, Nikov G, Krishnamoorthy V, Majumder PK, Kelliher MA. Targeting the Notch1 and mTOR pathways in a mouse T-ALL model. Blood. 2009 Jun 11;113(24):6172-81. doi: 10.1182/blood-2008-02-136762. Epub 2009 , Feb 26. PMID:19246562 doi:http://dx.doi.org/10.1182/blood-2008-02-136762
- ↑ Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008 Apr;9(4):377-83. doi: 10.1038/embor.2008.7. Epub 2008 Feb 15. PMID:18274550 doi:http://dx.doi.org/10.1038/embor.2008.7
- ↑ Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, Bhagat G, Agarwal AM, Basso G, Castillo M, Nagase S, Cordon-Cardo C, Parsons R, Zuniga-Pflucker JC, Dominguez M, Ferrando AA. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007 Oct;13(10):1203-10. doi: 10.1038/nm1636. Epub 2007 Sep 16. PMID:17873882 doi:http://dx.doi.org/10.1038/nm1636
- ↑ O'Neil J, Grim J, Strack P, Rao S, Tibbitts D, Winter C, Hardwick J, Welcker M, Meijerink JP, Pieters R, Draetta G, Sears R, Clurman BE, Look AT. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007 Aug 6;204(8):1813-24. doi: 10.1084/jem.20070876. Epub 2007 Jul, 23. PMID:17646409 doi:http://dx.doi.org/10.1084/jem.20070876
- ↑ Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010 Apr 15;464(7291):1052-7. PMID:20393564 doi:10.1038/nature08878
- ↑ Aste-Amezaga M, Zhang N, Lineberger JE, Arnold BA, Toner TJ, Gu M, Huang L, Vitelli S, Vo KT, Haytko P, Zhao JZ, Baleydier F, L'Heureux S, Wang H, Gordon WR, Thoryk E, Andrawes MB, Tiyanont K, Stegmaier K, Roti G, Ross KN, Franlin LL, Wang H, Wang F, Chastain M, Bett AJ, Audoly LP, Aster JC, Blacklow SC, Huber HE. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One. 2010 Feb 8;5(2):e9094. doi: 10.1371/journal.pone.0009094. PMID:20161710 doi:http://dx.doi.org/10.1371/journal.pone.0009094