1iyt
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /> <applet load="1iyt" size="450" color="white" frame="true" align="right" spinBox="true" caption="1iyt" /> '''Solution structure of the Alzheimer's disea...)
Next diff →
Revision as of 15:29, 12 November 2007
|
Solution structure of the Alzheimer's disease amyloid beta-peptide (1-42)
Contents |
Overview
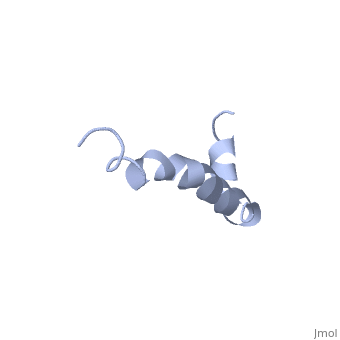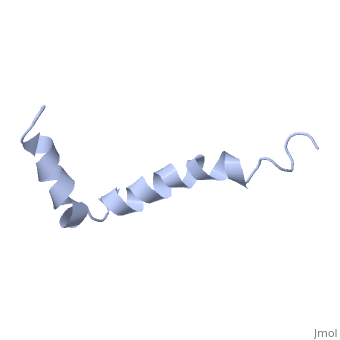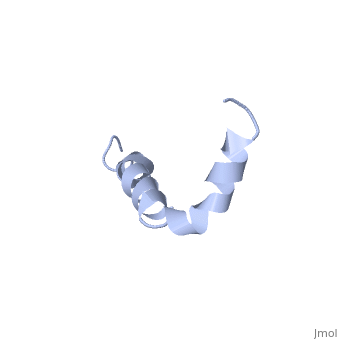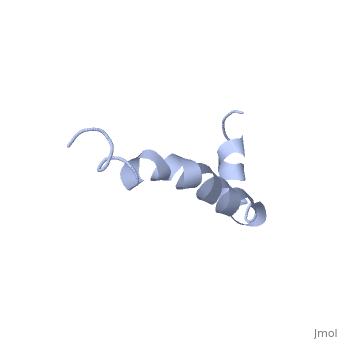
The major components of neuritic plaques found in Alzheimer disease (AD), are peptides known as amyloid beta-peptides (Abeta), which derive from the, proteolitic cleavage of the amyloid precursor proteins. In vitro Abeta may, undergo a conformational transition from a soluble form to aggregated, fibrillary beta-sheet structures, which seem to be neurotoxic., Alternatively, it has been suggested that an alpha-helical form can be, involved in a process of membrane poration, which would then trigger, cellular death. Conformational studies on these peptides in aqueous, solution are complicated by their tendency to aggregate, and only recently, NMR structures of Abeta-(1-40) and Abeta-(1-42) have been determined in, aqueous trifluoroethanol or in SDS micelles. All these studies hint to the, presence of two helical regions, connected through a flexible kink, but it, proved difficult to determine the length and position of the helical, stretches with accuracy and, most of all, to ascertain whether the kink, region has a preferred conformation. In the search for a medium which, could allow a more accurate structure determination, we performed an, exhaustive solvent scan that showed a high propensity of Abeta-(1-42) to, adopt helical conformations in aqueous solutions of fluorinated alcohols., The 3D NMR structure of Abeta-(1-42) shows two helical regions, encompassing residues 8-25 and 28-38, connected by a regular type I, beta-turn. The surprising similarity of this structure, as well as the, sequence of the C-terminal moiety, with those of the fusion domain of, influenza hemagglutinin suggests a direct mechanism of neurotoxicity.
Disease
Known diseases associated with this structure: Alzheimer disease-1, APP-related OMIM:[104760], Amyloidosis, cerebroarterial, Dutch type OMIM:[104760], Amyloidosis, cerebroarterial, Iowa type OMIM:[104760], Blood group, P system OMIM:[607922]
About this Structure
1IYT is a Single protein structure of sequence from [1]. The following page contains interesting information on the relation of 1IYT with [Amyloid-beta Precursor Protein]. Full crystallographic information is available from OCA.
Reference
Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain., Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D'Ursi AM, Temussi PA, Picone D, Eur J Biochem. 2002 Nov;269(22):5642-8. PMID:12423364
Page seeded by OCA on Mon Nov 12 17:36:04 2007