We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1767
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
SHOC2-PP1C-MRAS is a regulator of a cell proliferation pathway. Mutations in cell proliferation pathways are responsible for 25% of all cancers 1. If this cell proliferation pathway goes unmediated, rapid cell growth and division will occur; the leading cause of cancers is mutations in this pathway. [https://www.nature.com/articles/d41586-022-02189-7 Mechanistic Overview and Signaling Cascade ] shows the pathway SHOC2-PP1C-MRAS is part of. It is a receptor tyrosine kinase pathway 1. When the receptor binds, a phosphorylation causes a conformational change. This conformation change causes the phosphorylation of other residues. Eventually, this leads to the unbinding of GDP from MRAS and the binding of GTP to MRAS, causing a shift from the open to <scene name='95/952693/Switch_i_gtp_bound/10'> closed conformation of SWI.</scene>, The Switch I region is made up of residues 42-48 of the MRAS domain. 1 These residues are crucial for the binding of MRAS, SHOC2, and PP1C. When GDP is bound to the MRAS domain, it is in the <scene name='95/952693/Swi_open_conformation/3'>open conformation</scene>. Since the gamma P is not bound to GDP, there are no hydrogen bond interactions with the oxygens of the phosphate group- hence the open conformation. Figure 2 When GTP is bound to MRAS, it is in the closed conformation. The closed conformation allows for the binding of SHOC2 and PP1C. The open conformation of MRAS sterically clashes with the binding site of SHOC2, which is why the complex is not assembled when GDP is bound. The only large-scale conformational change occurs in the MRAS subunit. SHOC2 only undergoes a 6° <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/5'>conformational change</scene> Since SHOC2 and PP1C do not undergo much conformational change, they are in an equilibrium of binding and unbinding until MRAS-GTP binds to SHOC2, allowing complex assembly. | SHOC2-PP1C-MRAS is a regulator of a cell proliferation pathway. Mutations in cell proliferation pathways are responsible for 25% of all cancers 1. If this cell proliferation pathway goes unmediated, rapid cell growth and division will occur; the leading cause of cancers is mutations in this pathway. [https://www.nature.com/articles/d41586-022-02189-7 Mechanistic Overview and Signaling Cascade ] shows the pathway SHOC2-PP1C-MRAS is part of. It is a receptor tyrosine kinase pathway 1. When the receptor binds, a phosphorylation causes a conformational change. This conformation change causes the phosphorylation of other residues. Eventually, this leads to the unbinding of GDP from MRAS and the binding of GTP to MRAS, causing a shift from the open to <scene name='95/952693/Switch_i_gtp_bound/10'> closed conformation of SWI.</scene>, The Switch I region is made up of residues 42-48 of the MRAS domain. 1 These residues are crucial for the binding of MRAS, SHOC2, and PP1C. When GDP is bound to the MRAS domain, it is in the <scene name='95/952693/Swi_open_conformation/3'>open conformation</scene>. Since the gamma P is not bound to GDP, there are no hydrogen bond interactions with the oxygens of the phosphate group- hence the open conformation. Figure 2 When GTP is bound to MRAS, it is in the closed conformation. The closed conformation allows for the binding of SHOC2 and PP1C. The open conformation of MRAS sterically clashes with the binding site of SHOC2, which is why the complex is not assembled when GDP is bound. The only large-scale conformational change occurs in the MRAS subunit. SHOC2 only undergoes a 6° <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/5'>conformational change</scene> Since SHOC2 and PP1C do not undergo much conformational change, they are in an equilibrium of binding and unbinding until MRAS-GTP binds to SHOC2, allowing complex assembly. | ||
=== Ras/Raf === | === Ras/Raf === | ||
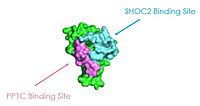
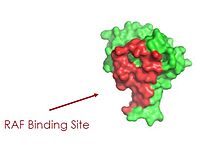
| - | [[Image:SHOC2.jpg|200 px| | + | As seen in the figures 1 and 2 below, the surface MRAS uses to recruit SHOC2 and PP1C is the same surface it uses to interact with Raf. As seen in these figures, there is a significant amount of steric overlap with MRAS binding site with PP1C and SHOC2 and Raf. Hence, multiple Ras proteins are required for further activation of the receptor tyrosine kinase pathway. The ability of Ras-GTP to cluster at the membrane is a crucial capability for this protein complex. This anchoring is possible due to the presence of a hydrophobic fatty acid tail on Ras. |
| + | [[Image:SHOC2.jpg|200 px|center|thumb|Figure 1]] | ||
Figure 1: MRAS binding sites to SHOC2 and PP1C. This image was created in PyMOL using surface view (PDB code 7DS0). | Figure 1: MRAS binding sites to SHOC2 and PP1C. This image was created in PyMOL using surface view (PDB code 7DS0). | ||
| + | [[Image:RAF.jpg|200 px|center|thumb|Figure 2]] | ||
| + | Figure 2: MRAS binding site to Raf. This image was created in PyMOL using surface view. (PDB code 7DSO). 3 | ||
== Structure of Active Site == | == Structure of Active Site == | ||
=== 3-Metal Ion Catalysis === | === 3-Metal Ion Catalysis === | ||
Revision as of 15:02, 3 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
SHOC2-PP1C-MRAS
| |||||||||||
Protopedia Resources
<protopedia resources/>
</StructureSection>
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
</StructureSection>
Student Contributors
<student contributors/>