Sandbox Reserved 1767
From Proteopedia
| Line 1: | Line 1: | ||
| - | {{Template:CH462_Biochemistry_II_2023}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | + | <scene name='95/952694/Overall_image/2'>SHOC2-PP1C-MRas Overall Structure</scene>{{Template:CH462_Biochemistry_II_2023}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> |
=SHOC2-PP1C-MRAS= | =SHOC2-PP1C-MRAS= | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| Line 18: | Line 18: | ||
=== SHOC2 === | === SHOC2 === | ||
=== PP1C === | === PP1C === | ||
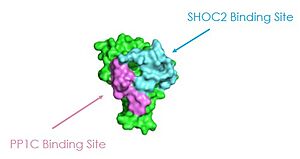
| + | SHOC2 has a RVxF binding motif, that binds to the PP1C RVxF binding site. The N-terminal loop of SHOC2 interacts with the RVxF binding site of PP1C, showing the structure and function connection of the complex. RVxF allows PP1C substrates to bind, whereas RAF has the RVxF motif, so it can bind to the hydrophobic region of SHOC2 which allows for greater specificity. Additionally, PP1C and SHOC2 do not change conformationally upon the binding of GTP, but rather they are inactive when RAS is bound to GDP due to steric strain. <scene name='95/952694/Pp1coverlay/1'>PP1C retains the same structure</scene> with or without binding to the SHOC2 complex. | ||
=== MRAS === | === MRAS === | ||
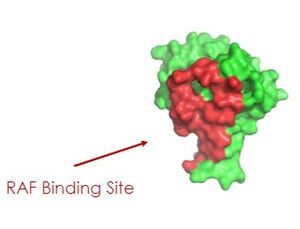
| + | MRas localized the SHOC2 complex to the cell membrane by its C-terminus end. In its <scene name='95/952694/Cell_membrane/2'>Cell Membrane Bound RAS Model</scene>, there is a modified chain that allows it to bind to the cell membrane. Normally, MRas does not have the chain and it is only added after the modification. For MRas to bind, the SHOC-2 complex must be in the GTP bound state. When GDP is bound, there is a steric clash between Switch 1 and PP1C, so interaction with MRAS is not possible. Additionally, the surface of MRas that is buried in the complex overlaps the surfaces used to engage RAF. It requires two MRas interactions to activate a single RAF molecule. | ||
| + | |||
=== Binding of the Subunits === | === Binding of the Subunits === | ||
| Line 27: | Line 30: | ||
=== Switch I and Switch II === | === Switch I and Switch II === | ||
SHOC2-PP1C-MRAS is a regulator of a cell proliferation pathway. Mutations in cell proliferation pathways are responsible for 25% of all cancers 1. If this cell proliferation pathway goes unmediated, rapid cell growth and division will occur; the leading cause of cancers is mutations in this pathway. [https://www.nature.com/articles/d41586-022-02189-7 Mechanistic Overview and Signaling Cascade ] shows the pathway SHOC2-PP1C-MRAS is part of. It is a receptor tyrosine kinase pathway 1. When the receptor binds, a phosphorylation causes a conformational change. This conformation change causes the phosphorylation of other residues. Eventually, this leads to the unbinding of GDP from MRAS and the binding of GTP to MRAS, causing a shift from the open to <scene name='95/952693/Switch_i_gtp_bound/10'> closed conformation of SWI.</scene>, The Switch I region is made up of residues 42-48 of the MRAS domain. 1 These residues are crucial for the binding of MRAS, SHOC2, and PP1C. When GDP is bound to the MRAS domain, it is in the <scene name='95/952693/Swi_open_conformation/3'>open conformation</scene>. Since the gamma P is not bound to GDP, there are no hydrogen bond interactions with the oxygens of the phosphate group- hence the open conformation. Figure 2 When GTP is bound to MRAS, it is in the closed conformation. The closed conformation allows for the binding of SHOC2 and PP1C. The open conformation of MRAS sterically clashes with the binding site of SHOC2, which is why the complex is not assembled when GDP is bound. The only large-scale conformational change occurs in the MRAS subunit. SHOC2 only undergoes a 6° <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/5'>conformational change</scene> Since SHOC2 and PP1C do not undergo much conformational change, they are in an equilibrium of binding and unbinding until MRAS-GTP binds to SHOC2, allowing complex assembly. | SHOC2-PP1C-MRAS is a regulator of a cell proliferation pathway. Mutations in cell proliferation pathways are responsible for 25% of all cancers 1. If this cell proliferation pathway goes unmediated, rapid cell growth and division will occur; the leading cause of cancers is mutations in this pathway. [https://www.nature.com/articles/d41586-022-02189-7 Mechanistic Overview and Signaling Cascade ] shows the pathway SHOC2-PP1C-MRAS is part of. It is a receptor tyrosine kinase pathway 1. When the receptor binds, a phosphorylation causes a conformational change. This conformation change causes the phosphorylation of other residues. Eventually, this leads to the unbinding of GDP from MRAS and the binding of GTP to MRAS, causing a shift from the open to <scene name='95/952693/Switch_i_gtp_bound/10'> closed conformation of SWI.</scene>, The Switch I region is made up of residues 42-48 of the MRAS domain. 1 These residues are crucial for the binding of MRAS, SHOC2, and PP1C. When GDP is bound to the MRAS domain, it is in the <scene name='95/952693/Swi_open_conformation/3'>open conformation</scene>. Since the gamma P is not bound to GDP, there are no hydrogen bond interactions with the oxygens of the phosphate group- hence the open conformation. Figure 2 When GTP is bound to MRAS, it is in the closed conformation. The closed conformation allows for the binding of SHOC2 and PP1C. The open conformation of MRAS sterically clashes with the binding site of SHOC2, which is why the complex is not assembled when GDP is bound. The only large-scale conformational change occurs in the MRAS subunit. SHOC2 only undergoes a 6° <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/5'>conformational change</scene> Since SHOC2 and PP1C do not undergo much conformational change, they are in an equilibrium of binding and unbinding until MRAS-GTP binds to SHOC2, allowing complex assembly. | ||
| + | |||
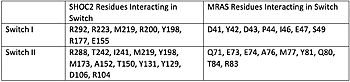
| + | [[Image:Table.jpeg|350 px|left|thumb|Figure 1. Residues Interacting at SWI and SWII at subunits SHOC2 and PP1C.]] | ||
| + | Switch I (SWI) and Switch II (SWII) are located between the SHOC2 and MRas subunits. When GTP is hydrolyzed to GDP, Switch I and Switch II relax, in the relaxed state SHOC2 cannot bind to MRas. Two Residues from MRas interact with the gamma phosphate on GTP, changing the complex to the closed confirmation. When GTP is bound to <scene name='95/952694/Mras_switch_i/1'>MRAS at SWI</scene>, it triggers the assembly of the SHOC2 Complex. When SWI is in its open confirmation, PP1C cannot bind with MRas due to the steric clashes, but when GTP binds and SWI is in its closed confirmation, PP1C can bind without hinderance. In a mutated complex, other RAS proteins can replace MRas making cell proliferation more likely. SHOC2-PP1C-MRas may be used as a therapeutic target for cancer treatments through changing the confirmation of the <scene name='95/952694/Mrasswitchii/1'>RAS SWII</scene>. | ||
| + | |||
=== Ras/Raf === | === Ras/Raf === | ||
As seen in the figures 1 and 2 below, the surface MRAS uses to recruit SHOC2 and PP1C is the same surface it uses to interact with Raf. As seen in these figures, there is a significant amount of steric overlap with MRAS binding site with PP1C and SHOC2 and Raf. Hence, multiple Ras proteins are required for further activation of the receptor tyrosine kinase pathway. The ability of Ras-GTP to cluster at the membrane is a crucial capability for this protein complex. This anchoring is possible due to the presence of a hydrophobic fatty acid tail on Ras. | As seen in the figures 1 and 2 below, the surface MRAS uses to recruit SHOC2 and PP1C is the same surface it uses to interact with Raf. As seen in these figures, there is a significant amount of steric overlap with MRAS binding site with PP1C and SHOC2 and Raf. Hence, multiple Ras proteins are required for further activation of the receptor tyrosine kinase pathway. The ability of Ras-GTP to cluster at the membrane is a crucial capability for this protein complex. This anchoring is possible due to the presence of a hydrophobic fatty acid tail on Ras. | ||
Revision as of 15:22, 3 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
SHOC2-PP1C-MRAS
| |||||||||||
Protopedia Resources
References
1. Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Mollé S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Sep;609(7926):416-423. doi: 10.1038/s41586-022-05086-1. Epub 2022 Jul 13. PMID: 35830882; PMCID: PMC9452295.[1].
2. Hurley TD, Yang J, Zhang L, Goodwin KD, Zou Q, Cortese M, Dunker AK, DePaoli-Roach AA. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007 Sep 28;282(39):28874-28883. doi: 10.1074/jbc.M703472200. Epub 2007 Jul 18. PMID: 17636256.[2].
3. Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Sep;609(7926):408-415. doi: 10.1038/s41586-022-04928-2. Epub 2022 Jul 13. PMID: 35831509; PMCID: PMC9694338.[3].
4. Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Sep;609(7926):400-407. doi: 10.1038/s41586-022-04838-3. Epub 2022 Jun 29. PMID: 35768504; PMCID: PMC9452301.[4].
5. Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. doi: 10.1038/d41586-022-02189-7. PMID: 35970881.[5].
6. Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. doi: 10.1073/pnas.1720352115. Epub 2018 Oct 22. PMID: 30348783; PMCID: PMC6233131.[6].
Student Contributors
- Sloan August
- Rosa Trippel
- Kayla Wilhoite