From Proteopedia
(Difference between revisions)
proteopedia linkproteopedia link
|
|
| Line 5: |
Line 5: |
| | | | |
| | == Introduction == | | == Introduction == |
| - | | + | <scene name='95/952694/Mrasswi/1'>MRAS SWI</scene> |
| | == Relevance == | | == Relevance == |
| | === Cell Proliferation === | | === Cell Proliferation === |
Revision as of 18:10, 3 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795.
|
To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
|
Structure
| This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
Introduction
Relevance
Cell Proliferation
Cancer
Structure of Subunits
SHOC2
PP1C
SHOC2 has a RVxF binding motif, that binds to the PP1C RVxF binding site. The N-terminal loop of SHOC2 interacts with the RVxF binding site of PP1C, showing the structure and function connection of the complex. RVxF allows PP1C substrates to bind, whereas RAF has the RVxF motif, so it can bind to the hydrophobic region of SHOC2 which allows for greater specificity. Additionally, PP1C and SHOC2 do not change conformationally upon the binding of GTP, but rather they are inactive when RAS is bound to GDP due to steric strain. with or without binding to the SHOC2 complex.[1].
MRAS
MRas localized the SHOC2 complex to the cell membrane by its C-terminus end. In its , there is a modified chain that allows it to bind to the cell membrane.[1] Normally, MRas does not have the chain and it is only added after the modification. For MRas to bind, the SHOC-2 complex must be in the GTP bound state. When GDP is bound, there is a steric clash between Switch 1 and PP1C, so interaction with MRAS is not possible. Additionally, the surface of MRas that is buried in the complex overlaps the surfaces used to engage RAF. It requires two MRas interactions to activate a single RAF molecule.
Binding of the Subunits
Signaling Cascade
Autoinhibited Confirmation
Ras/Raf
Switch I and Switch II
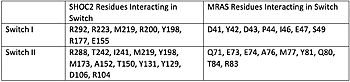 Figure 1. Residues Interacting at SWI and SWII at subunits SHOC2 and PP1C. [1]. Switch I (SWI) and Switch II (SWII) are located between the SHOC2 and MRas subunits. When GTP is hydrolyzed to GDP, Switch I and Switch II relax, in the relaxed state SHOC2 cannot bind to MRas. Two Residues from MRas interact with the gamma phosphate on GTP, changing the complex to the closed confirmation. When GTP is bound to , it triggers the assembly of the SHOC2 Complex. When SWI is in its open confirmation, PP1C cannot bind with MRas due to the steric clashes, but when GTP binds and SWI is in its closed confirmation, PP1C can bind without hinderance. In a mutated complex, other RAS proteins can replace MRas making cell proliferation more likely. SHOC2-PP1C-MRas may be used as a therapeutic target for cancer treatments through changing the confirmation of the .
Structure of Active Site
3-Metal Ion Catalysis
Hydrophobic Binding Site
Future Directions
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
References
- ↑ 1.0 1.1 1.2 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
1. Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Mollé S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Sep;609(7926):416-423. doi: 10.1038/s41586-022-05086-1. Epub 2022 Jul 13. PMID: 35830882; PMCID: PMC9452295.[1].
2. Hurley TD, Yang J, Zhang L, Goodwin KD, Zou Q, Cortese M, Dunker AK, DePaoli-Roach AA. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007 Sep 28;282(39):28874-28883. doi: 10.1074/jbc.M703472200. Epub 2007 Jul 18. PMID: 17636256.[2].
3. Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Sep;609(7926):408-415. doi: 10.1038/s41586-022-04928-2. Epub 2022 Jul 13. PMID: 35831509; PMCID: PMC9694338.[3].
4. Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Sep;609(7926):400-407. doi: 10.1038/s41586-022-04838-3. Epub 2022 Jun 29. PMID: 35768504; PMCID: PMC9452301.[4].
5. Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. doi: 10.1038/d41586-022-02189-7. PMID: 35970881.[5].
6. Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. doi: 10.1073/pnas.1720352115. Epub 2018 Oct 22. PMID: 30348783; PMCID: PMC6233131.[6].

