Sandbox Reserved 1791
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
== Active vs. Inactive State == | == Active vs. Inactive State == | ||
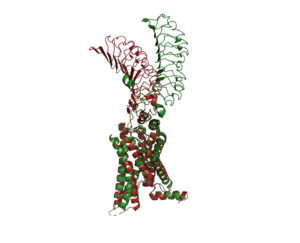
| - | + | [[Image:Image-Inactive Inactive Proteopedia.png|300 px|right|thumb| An overview of the Inactive (red) vs Active (green) state of TSHR. PDB: 7WX5]] | |
The <scene name='95/952719/Active_form/4'>active form</scene> of TSHR is found when bound by TSH or M22. The structure can be seen as straight. The <scene name='95/952719/Inactive_form/6'>inactive form</scene> of TSHR is found when bound with K1. The overall structure of the molecule is bent when K1 is bound. When TSHR is not bound to TSH, it is in the inactive state. This is also considered the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashing between TSH and the cell-membrane cause TSHR to take on the active or "up" state. During this transition, the Extracellular domains rotate 55° along an axis. This rotation is caused by conformational changes within the <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>, specifically at the <scene name='95/952719/Hinge_region_residues/3'>Y279</scene> residue. This residue moves 6 angstroms relative to I486, which is a residue located in the Transmembrane Region <ref name="Faust"/> | The <scene name='95/952719/Active_form/4'>active form</scene> of TSHR is found when bound by TSH or M22. The structure can be seen as straight. The <scene name='95/952719/Inactive_form/6'>inactive form</scene> of TSHR is found when bound with K1. The overall structure of the molecule is bent when K1 is bound. When TSHR is not bound to TSH, it is in the inactive state. This is also considered the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashing between TSH and the cell-membrane cause TSHR to take on the active or "up" state. During this transition, the Extracellular domains rotate 55° along an axis. This rotation is caused by conformational changes within the <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>, specifically at the <scene name='95/952719/Hinge_region_residues/3'>Y279</scene> residue. This residue moves 6 angstroms relative to I486, which is a residue located in the Transmembrane Region <ref name="Faust"/> | ||
Revision as of 15:00, 7 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ doi: https://dx.doi.org/https
- ↑ Smits G, Govaerts C, Nubourgh I, Pardo L, Vassart G, Costagliola S. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol. 2002 Apr;16(4):722-35. PMID:11923469 doi:10.1210/mend.16.4.0815
- ↑ 5.0 5.1 Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther. 2019 Sep;36(Suppl 2):47-58. PMID:31485975 doi:10.1007/s12325-019-01080-8
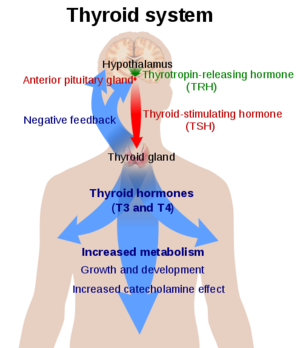
![(Fig. 3) TSH’s role in the diagnosis of Hyperthyroidism and Hypothyroidism: [1]](/wiki/images/thumb/9/95/TSH_role.jpeg/400px-TSH_role.jpeg)
