Introduction
Thyroid Stimulating Hormone Receptor (TSHR) is a type of G-Protein Coupled Receptor (GPCR) found in human thyroid follicles. It is activated by the Thyroid Stimulating Hormone (TSH) which is known as thyrotropin. Activation of TSHR is necessary for activating a signaling pathway for the production of thyroid hormones such as triiodothyronine(T3) and thyroxine(T4)
Structure
The structure of TSHR and the antibodies bound were found using cyro-EM. TSHR forms a complex with TSH and Gs proteins. This is called the .
: Leucine Rich Region Domain (coral), the hinge region (blue-purple), and the transmembrane region(rainbow). The leucine rich region domain is located on the outside of the cell. This is where TSH will bind. The hinge region is also extracellular. Conformational changes in this region are responsible for the switch between the active vs inactive state. Finally, the transmembrane region is located within the plasma membrane. Its function is to hold the receptor into the membrane. This domain is also bound to the G-proteins at the N-terminus.
Transmembrane Region
() is embedded within the cell membrane. Like other G-proteins, it is made up of a 7-pass helix [1]. The transmembrane region is surrounded by a "belt" of . When cholesterol binding sites are mutated such that they are unfunctional, TSHR activity decreases. Thus, the cholesterols are important for TSHR function (reference). Additionally, at the N-terminus, the transmembrane region binds to the .
Hinge Region
The (purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. It is made up of two α-helices that are connected via disulfide bonds(shown in yellow). Interactions between these two helices and TSH help orient TSH properly. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. Conformational changes in this region, specifically the orientation of , are responsible for bringing TSHR into the active state [1].
Leucine Rich Domain
The is part of the extracellular region of TSHR. Connected to its C-terminus is the Hinge Region. It is made up of an extensive parallel β-sheet. This β-sheet is where TSH binds and is called the binding pocket[2].
Binding Pocket
The is a concave structure with many polar residues. This pocket is where the TSH antibody and agonist K1 bind as well as the agonist M22. These structures interact with specific residues to result in a structural change of the molecule. There is a mutation done by N-glycans at asparagine residues that plays a large role in the binding of TSH. The negative charge on these glycans contributes to the polarity of the binding pocket which mediates the binding efficiency of TSH. It has been shown that four of the five N glycan sites must be glycosylated to be in the active form. [3].
Active vs. Inactive State
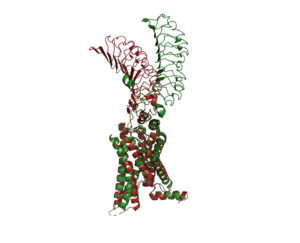
An overview of the Inactive (red) vs Active (green) state of TSHR. PDB: 7WX5
When TSHR is not bound to TSH, it is in the . This is also considered the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashing between TSH and the cell-membrane cause TSHR to take on the . During this transition, the Extracellular domains rotate 55° along an axis. This rotation is caused by conformational changes within the , specifically at the . This residue moves 6 angstroms relative to I486, which is a residue located in the Transmembrane Region [1]
The active form is found when . The structure can be seen as straight. The inactive form is found when of TSHR is found when bound with K1. The overall structure of the molecule is bent when K1 is bound. When TSHR is not bound to TSH, it is in the inactive state. This is also considered the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashing between TSH and the cell-membrane cause TSHR to take on the active or "up" state. During this transition, the Extracellular domains rotate 55° along an axis. This rotation is caused by conformational changes within the , specifically at the residue. This residue moves 6 angstroms relative to I486, which is a residue located in the Transmembrane Region [1]
Specific Residues and Interactions
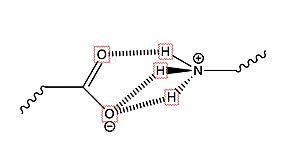
(Fig.2) Salt bridge interaction between LYS and Glu or Asp
There are two in the binding pocket of TSHR that are the main contributors to the binding of the antibodies. The concave structure of the binding pocket allows a with the antibodies. These antibodies make tight interactions with a lot of intermolecular forces at play. interacts with Glu 118 on the antibodies to make a salt bridge interaction. interacts with Asp 11 on the antibodies to make a salt bridge interaction. Specifically, the two residues make an ionic interaction. The interaction is not close enough to make a hydrogen bond. Instead, the interaction between the Lys residues with the Asp or Glu residues is a salt bridge interaction. This is the main bond that holds these two molecules together. When in the inactive form, LYS 209 does not interact with any residue but LYS 58 has interaction with Glu 118 and this interaction pulls the molecule into the bent position. The salt bridge interaction between Lys and Glu is very specific. Lys was mutated with Arg and was expected to make the same salt bride interaction with Glu, however it make a completely different interaction. This new interaction favors a gain of function towards hCG [4]
Biological Relevance
![(Fig. 3) TSH’s role in the diagnosis of Hyperthyroidism and Hypothyroidism: [1]](/wiki/images/thumb/9/95/TSH_role.jpeg/400px-TSH_role.jpeg)
(Fig. 3) TSH’s role in the diagnosis of Hyperthyroidism and Hypothyroidism:
[1]The thyroid plays an essential role in the body's metabolism. The body's metabolism affects things like heart rate, digestion, temperature regulation, and many more things. When TSH is bound to TSHR, a signal is sent to produce T3 and T4. Those hormones then go impact cells in your body to increase or decrease your metabolism.
Hyperthyroidism
Hyperthyroidism is when the thyroid is overactive. When the M22 antibody is bound it keeps TSHR in the active conformation. This overstimulates the body's metabolism. Symptoms of hyperthyroidism include but are not limited to fast or irregular heartbeats, tiredness, increased hunger, sleep problems, enlarged thyroid gland, and sensitivity to heat. Grave's Disease is the most common cause of hyperthyroidism. This is an autoimmune disorder that causes your body to attack the thyroid gland[5].
Hypothyroidism
Hypothyroidism is when the thyroid is underactive. When the K1 antibody is bound it does not allow the TSHR to go into the upright, active, position. This does not allow for the signaling of the T3 and T4 hormones to upregulate the metabolism. The symptoms of this include slow or irregular heartbeats, tiredness, muscle aches, memory problems, jaundice, and sensitivity to cold. Hasimoto's disease is an example of hypothyroidism. This is an autoimmune disorder that causes your body to attack the healthy cells of the thyroid. Specifically causing the death of the cells that produce the thyroid hormones. When the thyroid fails to produce its hormones it activates TSH production through a negative feedback mechanism[5]
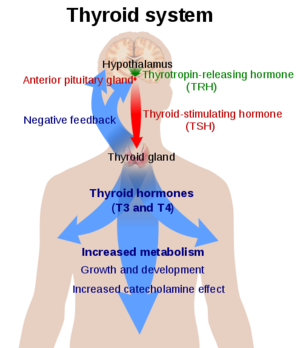


![(Fig. 3) TSH’s role in the diagnosis of Hyperthyroidism and Hypothyroidism: [1]](/wiki/images/thumb/9/95/TSH_role.jpeg/400px-TSH_role.jpeg)
