We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1788
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
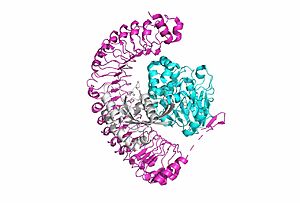
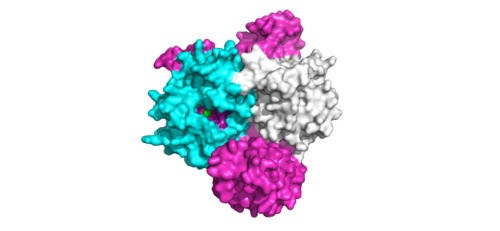
<scene name='95/952717/Shoc2_and_pp1c/1'>PP1C binds to SHOC2</scene> on its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main <scene name='95/952717/Shoc2_and_pp1c/2'>hydrogen bonds</scene> are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on <scene name='95/952718/Acid_base_pp1c/1'>PP1C</scene> and <scene name='95/952718/Acid_base_shoc2/1'>SHOC2</scene>. The corresponding patches interact to form a <scene name='95/952718/Acid_base_shoc2pp1c/1'>binary complex</scene>. These interactions do not result in significant conformational changes. | <scene name='95/952717/Shoc2_and_pp1c/1'>PP1C binds to SHOC2</scene> on its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main <scene name='95/952717/Shoc2_and_pp1c/2'>hydrogen bonds</scene> are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on <scene name='95/952718/Acid_base_pp1c/1'>PP1C</scene> and <scene name='95/952718/Acid_base_shoc2/1'>SHOC2</scene>. The corresponding patches interact to form a <scene name='95/952718/Acid_base_shoc2pp1c/1'>binary complex</scene>. These interactions do not result in significant conformational changes. | ||
| + | |||
| + | <scene name='95/952716/Shoc2_and_pp1c/3'>TextToBeDisplayed</scene> | ||
== SHOC2 and MRAS == | == SHOC2 and MRAS == | ||
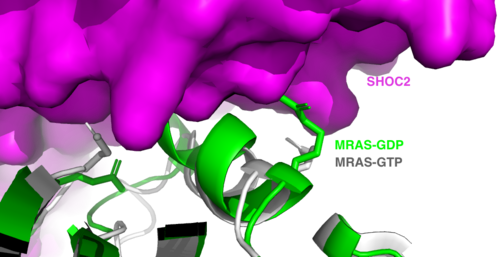
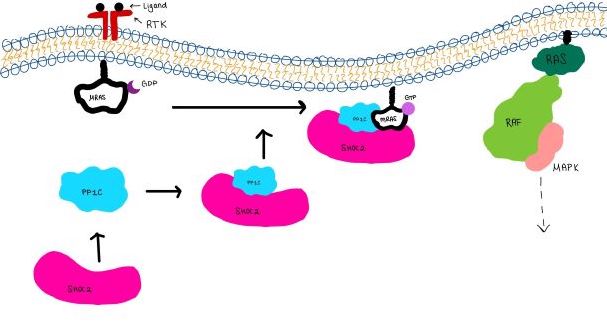
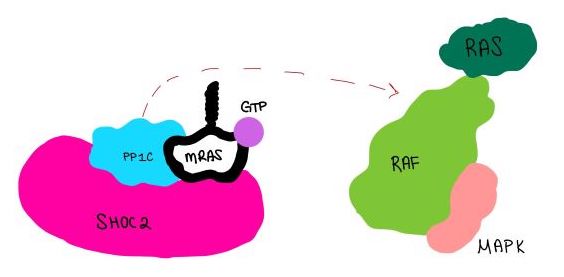
MRAS is initially bound to GDP causing it to be in its inactive state. This form cannot bind to the SHOC2-PP1C complex due to steric clashing (Figure 2). Once GDP is exchanged for GTP to activate the protein, <scene name='95/952716/conformational changes/1'>SHOC2-MRAS (residues)</scene> occur within the switch I and switch II regions to allow <scene name='95/952716/MRAS to interact with SHOC2/2'>SHOC2-MRAS(full-image)</scene>. These <scene name='95/952716/Scho2-mras-interactions/1'>interactions</scene> include hydrogen bonds and pi stacking. The primary hydrogen bonds are R288-Q71 and R177-E47. Pi staking occurs at R104-R83. | MRAS is initially bound to GDP causing it to be in its inactive state. This form cannot bind to the SHOC2-PP1C complex due to steric clashing (Figure 2). Once GDP is exchanged for GTP to activate the protein, <scene name='95/952716/conformational changes/1'>SHOC2-MRAS (residues)</scene> occur within the switch I and switch II regions to allow <scene name='95/952716/MRAS to interact with SHOC2/2'>SHOC2-MRAS(full-image)</scene>. These <scene name='95/952716/Scho2-mras-interactions/1'>interactions</scene> include hydrogen bonds and pi stacking. The primary hydrogen bonds are R288-Q71 and R177-E47. Pi staking occurs at R104-R83. | ||
| - | <scene name='95/952716/Shoc2_and_pp1c/3'>TextToBeDisplayed</scene> | ||
[[Image:Switches.png|500 px|thumb|'''Figure 2:'''Steric clashing of Switch I and II of GDP bound MRAS, in green, with the surface of SHOC2, in magenta. GTP-bound MRAS, in white, shows no steric clashing with SHOC2s surface.</div></font>]] | [[Image:Switches.png|500 px|thumb|'''Figure 2:'''Steric clashing of Switch I and II of GDP bound MRAS, in green, with the surface of SHOC2, in magenta. GTP-bound MRAS, in white, shows no steric clashing with SHOC2s surface.</div></font>]] | ||
Revision as of 16:40, 7 April 2023
| |||||||||||