Sandbox Reserved 1791
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
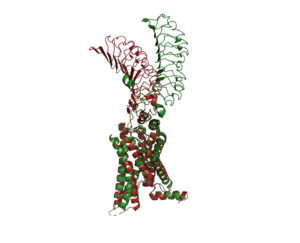
<scene name='95/952720/Transmembrane_region_spin/5'>The Transmembrane Region</scene> (<scene name='95/952720/Transmembrane_region_top-view/2'>top-view</scene>) is embedded within the cell membrane. Like other G-protein receptors, it is made up of a 7-pass helix <ref name="Faust"> DOI 10.1038/s41586-022-05159-1</ref>. It is made up of about 284 residues. The transmembrane region is surrounded by a "belt" of <scene name='95/952719/Tmd_cholesterol_spin/2'>15 cholesterols</scene>. When cholesterol binding sites are mutated such that they are unfunctional, TSHR activity decreases. Thus, the cholesterols are important for TSHR function <ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. Additionally, at the N-terminus, the transmembrane region binds to the <scene name='95/952720/Transmembrane_region_spin/4'>G-proteins</scene>, which are located intracellularly. | <scene name='95/952720/Transmembrane_region_spin/5'>The Transmembrane Region</scene> (<scene name='95/952720/Transmembrane_region_top-view/2'>top-view</scene>) is embedded within the cell membrane. Like other G-protein receptors, it is made up of a 7-pass helix <ref name="Faust"> DOI 10.1038/s41586-022-05159-1</ref>. It is made up of about 284 residues. The transmembrane region is surrounded by a "belt" of <scene name='95/952719/Tmd_cholesterol_spin/2'>15 cholesterols</scene>. When cholesterol binding sites are mutated such that they are unfunctional, TSHR activity decreases. Thus, the cholesterols are important for TSHR function <ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. Additionally, at the N-terminus, the transmembrane region binds to the <scene name='95/952720/Transmembrane_region_spin/4'>G-proteins</scene>, which are located intracellularly. | ||
| - | + | ||
| - | + | ||
=== Leucine Rich Domain=== | === Leucine Rich Domain=== | ||
The <scene name='95/952719/Lrrd/1'>Leucine Rich Repeat Domain (LRRD)</scene> is part of the extracellular region of TSHR. It is made up of about 280 different residues. Connected to its C-terminus is the Hinge Region. It is made up of an extensive parallel β-sheet. This β-sheet is where TSH binds and is called the binding pocket<ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. | The <scene name='95/952719/Lrrd/1'>Leucine Rich Repeat Domain (LRRD)</scene> is part of the extracellular region of TSHR. It is made up of about 280 different residues. Connected to its C-terminus is the Hinge Region. It is made up of an extensive parallel β-sheet. This β-sheet is where TSH binds and is called the binding pocket<ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. | ||
| + | |||
| + | === Hinge Region=== | ||
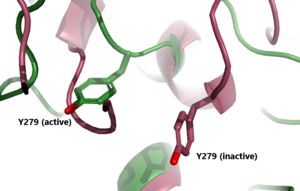
| + | The <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>(purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. It is sometimes referred to as the signaling specificity domain because there is some evidence suggesting that this region is important in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. It is made up of two α-helices that are connected via disulfide bonds(shown in yellow). Interactions between these two helices and TSH help orient TSH properly. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. Conformational changes in this region, specifically the orientation of <scene name='95/952719/Hinge_region_residues/3'>Y279</scene>, are responsible for bringing TSHR into the active state <ref name="Faust"/>. | ||
=== Binding Pocket=== | === Binding Pocket=== | ||
Revision as of 17:37, 7 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 2.0 2.1 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]
- ↑ Fokina, E.F., Shpakov, A.O. Thyroid-Stimulating Hormone Receptor: the Role in the Development of Thyroid Pathology and Its Correction. J Evol Biochem Phys 58, 1439–1454 (2022). [DOI:10.1134/S0022093022050143 https://doi.org/10.1134/S0022093022050143]
- ↑ Smits G, Govaerts C, Nubourgh I, Pardo L, Vassart G, Costagliola S. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol. 2002 Apr;16(4):722-35. doi: 10.1210/mend.16.4.0815. PMID: 11923469. [DOI: 10.1210/mend.16.4.0815 https://pubmed.ncbi.nlm.nih.gov/11923469/]
- ↑ 6.0 6.1 Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther. 2019 Sep;36(Suppl 2):47-58. doi: 10.1007/s12325-019-01080-8. Epub 2019 Sep 4. PMID: 31485975; PMCID: PMC6822815. [DOI: 10.1007/s12325-019-01080-8 https://pubmed.ncbi.nlm.nih.gov/31485975/]
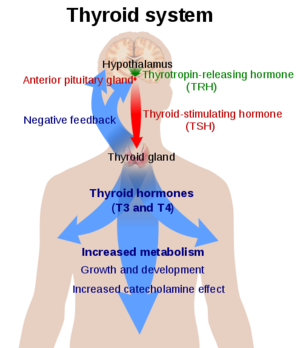
![(Fig. 3) TSH’s role in the diagnosis of Hyperthyroidism and Hypothyroidism: [1]](/wiki/images/thumb/9/95/TSH_role.jpeg/400px-TSH_role.jpeg)
