Sandbox Reserved 1767
From Proteopedia
| Line 13: | Line 13: | ||
== Structure of Subunits == | == Structure of Subunits == | ||
=== SHOC2 === | === SHOC2 === | ||
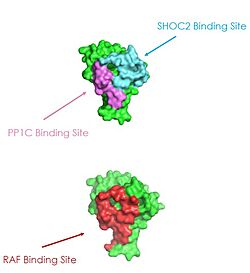
| - | SHOC2 is essential for complex formation, | + | The presence of SHOC2 is essential for complex formation. It a crescent shaped complex that serves as a bridge for PP1C and MRAS, maximizing interaction between the three subunits of the SMP complex. SHOC2 contains a large leucine rich region (LRR) that provides stability and localizes subunit PP1C to the membrane. Houseman SHOC2 only undergoes a 6° conformational change when PP1C and MRAS bind, showing SHOC2 is a scaffolding protein that provides a favorable interface for complex formation. SHOC2 depletion is being studied as a therapeutic approach for RAS-driven cancers due to large scale interactions of the subunits only being made possible due to SHOC2. Hauseman. SHOC2 and PP1C first engage in binding with each other via an N-terminal RVXF motif on SHOC2 that is complimentary to a sequence on PP1C. SHOC2 residues V64 and F66 embed in the complimentary region of PP1C, enhancing SHOC2 affinity for PP1C. SHOC2 bind MRAS-GTP through B strands of a LRR that interacts with a hydrophobic region of MRAS-GTP further stabilizing the complex. KWON |
=== PP1C === | === PP1C === | ||
| - | The | + | The Protein phosphatase complex 1 (PP1C) subunit contains the catalytic site of the SMP complex. The PP1C subunit is a phosphatase enzyme responsible for the removal of a phosphate group on an N-terminal phosphoserine (NTpS) on RAF. .<ref name="Liau">PMID: 35768504</ref> This dephosphorylation event allows for pathway activation. PP1C binds to SHOC2 and MRAS-GTP in a specific orientation that leaves this catalytic site accessible for substrate binding. PP1C cannot act independently from the SMP complex because it lacks intrinsic substrate selectivity.<ref name="Liau">PMID: 35768504</ref> Therefore, PP1C requires the presence of SHOC2 and MRAS to function properly, whereas SHOC2 and MRAS may interact in a binary complex without the presence of PP1C <ref name="Hauseman">PMID:35830882</ref> SMP complex formation is initially mediated by SHOC2 and PP1C, then stabilized by the GTP interactions in MRAS and SHOC2.<ref name="Kwon">PMID: 35831509</ref> Similarly to SHOC2, PP1C does not undergo a significant conformational change when SHOC2 and MRAS-GTP bind. The lack of conformational change shows that the structure of PP1C is not dependent on the SMP complex, but in order to act as a phosphatase it must be bound to the complex.<ref name="Liau">PMID: 35768504</ref>. PP1C is involved in many different cellular signaling pathways including protein synthesis, muscle contraction, and even carbohydrate metabolism. Wolfgang It plays an essential role in regulation of many pathways, not just cell proliferation. In all these pathways, including the SMP pathway, PP1C does not exist as a monomer, it is present in holoenzyme form complex with one of two regulatory subunits ensuring there is no sporadic pathway activation. Schulman |
| - | + | ||
| - | PP1C | + | |
| - | + | ||
=== MRAS === | === MRAS === | ||
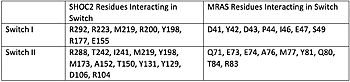
Ras proteins are GTP dependent switches that are associated with the plasma membrane.<ref name="Liau">PMID: 35768504</ref> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555610/ Ras proteins] often regulate cycles during signal transduction. MRAS, one of the subunits in the SMP complex, is a RAS protein specific to SHOC2 and the SMP complex. Other RAS proteins may bind to SHOC2, but MRAS induces the complex formation with a significantly lower Kd (dissociation constant), thus has the strongest connection.<ref name="Liau">PMID: 35768504</ref> The interface between SHOC2 and MRAS consists of two switches, Switch I and Switch II.<ref name="Liau">PMID: 35768504</ref> The switch regions were the only regions in MRAS to conformationally change, depending on the state of GTP.<ref name="Liau">PMID: 35768504</ref> | Ras proteins are GTP dependent switches that are associated with the plasma membrane.<ref name="Liau">PMID: 35768504</ref> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555610/ Ras proteins] often regulate cycles during signal transduction. MRAS, one of the subunits in the SMP complex, is a RAS protein specific to SHOC2 and the SMP complex. Other RAS proteins may bind to SHOC2, but MRAS induces the complex formation with a significantly lower Kd (dissociation constant), thus has the strongest connection.<ref name="Liau">PMID: 35768504</ref> The interface between SHOC2 and MRAS consists of two switches, Switch I and Switch II.<ref name="Liau">PMID: 35768504</ref> The switch regions were the only regions in MRAS to conformationally change, depending on the state of GTP.<ref name="Liau">PMID: 35768504</ref> | ||
Revision as of 14:08, 12 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
SHOC2-PP1C-MRAS
| |||||||||||
Protopedia Resources
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-04928-2. doi:, 10.1038/s41586-022-04928-2. PMID:35831509 doi:http://dx.doi.org/10.1038/s41586-022-04928-2
- ↑ 2.0 2.1 2.2 2.3 2.4 Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Molle S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-05086-1. doi:, 10.1038/s41586-022-05086-1. PMID:35830882 doi:http://dx.doi.org/10.1038/s41586-022-05086-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 4.0 4.1 Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. PMID:30348783 doi:10.1073/pnas.1720352115
- ↑ 5.0 5.1 5.2 Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. PMID:35970881 doi:10.1038/d41586-022-02189-7
- ↑ 6.0 6.1 6.2 6.3 Hurley TD, Yang J, Zhang L, Goodwin KD, Zou Q, Cortese M, Dunker AK, DePaoli-Roach AA. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007 Sep 28;282(39):28874-83. Epub 2007 Jul 18. PMID:17636256 doi:http://dx.doi.org/10.1074/jbc.M703472200
1. Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Mollé S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Sep;609(7926):416-423. doi: 10.1038/s41586-022-05086-1. Epub 2022 Jul 13. PMID: 35830882; PMCID: PMC9452295.[1].
2. Hurley TD, Yang J, Zhang L, Goodwin KD, Zou Q, Cortese M, Dunker AK, DePaoli-Roach AA. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007 Sep 28;282(39):28874-28883. doi: 10.1074/jbc.M703472200. Epub 2007 Jul 18. PMID: 17636256.[2].
3. Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Sep;609(7926):408-415. doi: 10.1038/s41586-022-04928-2. Epub 2022 Jul 13. PMID: 35831509; PMCID: PMC9694338.[3].
4. Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Sep;609(7926):400-407. doi: 10.1038/s41586-022-04838-3. Epub 2022 Jun 29. PMID: 35768504; PMCID: PMC9452301.[4].
5. Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. doi: 10.1038/d41586-022-02189-7. PMID: 35970881.[5].
6. Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. doi: 10.1073/pnas.1720352115. Epub 2018 Oct 22. PMID: 30348783; PMCID: PMC6233131.[6].
Student Contributors
- Sloan August
- Rosa Trippel
- Kayla Wilhoite