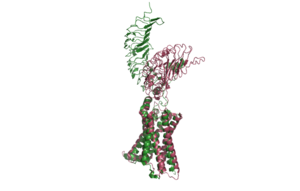We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1792
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
TSHR forms an active signalling complex with TSH and G<sub>s</sub> proteins. This is called the <scene name=scene name='95/952720/Tsh-tshr-gs_complex/3'>TSH-TSHR-Gs Complex</scene>. TSH contains an α and a β subunit. The α subunit is a shared subunit amongst glycoproteins. The β subunit is unique to TSH. TSH binds to the extracellular domain of TSHR <ref name="Duan"/> DOI 10.1038/s41586-022-05173-3</ref>. | TSHR forms an active signalling complex with TSH and G<sub>s</sub> proteins. This is called the <scene name=scene name='95/952720/Tsh-tshr-gs_complex/3'>TSH-TSHR-Gs Complex</scene>. TSH contains an α and a β subunit. The α subunit is a shared subunit amongst glycoproteins. The β subunit is unique to TSH. TSH binds to the extracellular domain of TSHR <ref name="Duan"/> DOI 10.1038/s41586-022-05173-3</ref>. | ||
<scene name=scene name='95/952720/Structure_overview_spins/3'> | <scene name=scene name='95/952720/Structure_overview_spins/3'> | ||
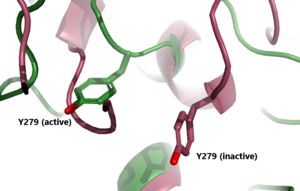
| - | TSHR has 3 main domains</scene>: Leucine Rich Region Domain (coral), the hinge region (blue-purple), and the transmembrane region(rainbow). The leucine rich region domain is extracellular | + | TSHR has 3 main domains</scene>: Leucine Rich Region Domain (coral), the hinge region (blue-purple), and the transmembrane region(rainbow). The leucine rich region domain is the extracellular TSH ligand domain. The hinge connects the Leucine Rich Repeat Domain and the Transmembrane Region. It provides flexibility for the switch between the active and inactive state of TSHR. The transmembrane region is located within the plasma membrane. Its function transmit the extracellular signal across the membrane to the intracellular [https://en.wikipedia.org/wiki/G_protein G-proteins] bound to the N-terminus of the transmembrane region<ref name="Duan"/>. Activated G-proteins then signal a robust intracellular signaling cascade. |
| - | + | ||
=== Transmembrane Region=== | === Transmembrane Region=== | ||
| - | <scene name='95/952720/Transmembrane_region_spin/5'>The Transmembrane Region</scene> (<scene name='95/952720/Transmembrane_region_top-view/2'>top-view</scene>) is embedded within the cell membrane | + | <scene name='95/952720/Transmembrane_region_spin/5'>The Transmembrane Region</scene> (<scene name='95/952720/Transmembrane_region_top-view/2'>top-view</scene>) is embedded within the cell membrane, like other G-protein receptors, it is composed of a 7-pass helix <ref name="Faust"/> PMID:35940205</ref>. The transmembrane region is surrounded by a "belt" of <scene name='95/952720/Tmd_cholesterol_spin/2'>15 cholesterols</scene><ref name = "Duan"> DOI 10.1038/s41586-022-05173-3</ref>. When cholesterol binding sites are mutated, TSHR activity decreases. These cholesterols are likely important for TSHR function <ref name="Duan"/>. Additionally, at the N-terminus, the transmembrane region binds to the <scene name='95/952720/Transmembrane_g-protein/1'>G-protein</scene>, which are located intracellularly <ref name="GOEL"/>. The G-proteins are made up of three subunits: α,β, and γ. When TSHR is activated, it causes the Gα subunit to dissociate from the Gβγ subunits. The Gα subunit is responsible for activating [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylyl cyclase], [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] and [https://en.wikipedia.org/wiki/Ion_channel ion channels]. This sets off the robust intracellular signaling cascade<ref name="GOEL"> PMID:24255551</ref>. |
| + | |||
| - | === Leucine Rich Domain=== | + | === Leucine Rich Domain===αα |
The <scene name='95/952720/Lrrd/1'>Leucine Rich Repeat Domain (LRRD)</scene> is part of the extracellular region of TSHR. It is made up of about 280 different residues. Connected to its C-terminus is the Hinge Region. It is made up of an extensive parallel β-sheet. This β-sheet is where TSH binds and is called the binding pocket <ref name="Duan"/>. | The <scene name='95/952720/Lrrd/1'>Leucine Rich Repeat Domain (LRRD)</scene> is part of the extracellular region of TSHR. It is made up of about 280 different residues. Connected to its C-terminus is the Hinge Region. It is made up of an extensive parallel β-sheet. This β-sheet is where TSH binds and is called the binding pocket <ref name="Duan"/>. | ||
Revision as of 17:52, 14 April 2023
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 3.3 3.4 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedFaust - ↑ 5.0 5.1 Goel R, Raju R, Maharudraiah J, Sameer Kumar GS, Ghosh K, Kumar A, Lakshmi TP, Sharma J, Sharma R, Balakrishnan L, Pan A, Kandasamy K, Christopher R, Krishna V, Mohan SS, Harsha HC, Mathur PP, Pandey A, Keshava Prasad TS. A Signaling Network of Thyroid-Stimulating Hormone. J Proteomics Bioinform. 2011 Oct 29;4:10.4172/jpb.1000195. PMID:24255551 doi:10.4172/jpb.1000195
- ↑ Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]
Student Contributors
- Alex Kem
- Grace Lane