We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1767
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
In all images and animations, {{Font color|cyan|SHOC2}} will be shown as cyan blue, {{Font color|lime|MRAS}} as lime, and {{Font color|violet|PP1C}} as violet. Other important components involved in the function of the SMP complex include the {{Font color|salmon|14-3-3}} dimer and {{Font color|slateblue|Raf}}, which will be shown in salmon and slate-blue, respectively. | In all images and animations, {{Font color|cyan|SHOC2}} will be shown as cyan blue, {{Font color|lime|MRAS}} as lime, and {{Font color|violet|PP1C}} as violet. Other important components involved in the function of the SMP complex include the {{Font color|salmon|14-3-3}} dimer and {{Font color|slateblue|Raf}}, which will be shown in salmon and slate-blue, respectively. | ||
| - | === | + | ===SMP Complex Mechanism=== |
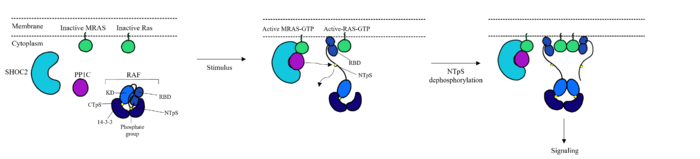
[[Image:MECH.png|700 px|center|thumb|'''Figure 1''': Mechanism of SMP complex formation and activation of RAF.<ref name="Liau">PMID: 35768504</ref><ref name="Lavoie">PMID: 35970881</ref>]] | [[Image:MECH.png|700 px|center|thumb|'''Figure 1''': Mechanism of SMP complex formation and activation of RAF.<ref name="Liau">PMID: 35768504</ref><ref name="Lavoie">PMID: 35970881</ref>]] | ||
| - | The Ras-Raf signaling cascade is inhibited when Raf is phosphorylated at Ser259. There is a <scene name='95/952695/14-3-3/1'>14-3-3</scene> dimer present in the cytoplasm that interacts with Raf through hydrogen bonds between R129 of 14-3-3 and Ser259 of Raf when Ser259 is phosphorylated. This interaction causes an <scene name='95/952695/Autoinhibited_confirmation/8'>autoinhibited confirmation</scene> as 14-3-3 restricts Raf to the cytoplasm and sterically inhibits Raf from binding with | + | The Ras-Raf signaling cascade is inhibited when Raf is phosphorylated at Ser259.<ref name="Kwon">PMID: 35831509</ref> There is a <scene name='95/952695/14-3-3/1'>14-3-3</scene> dimer present in the cytoplasm that interacts with Raf through hydrogen bonds between R129 of 14-3-3 and Ser259 of Raf when Ser259 is phosphorylated. This interaction causes an <scene name='95/952695/Autoinhibited_confirmation/8'>autoinhibited confirmation</scene> as 14-3-3 restricts Raf to the cytoplasm and sterically inhibits Raf from binding with Ras. This interaction is crucial in regulating [https://us.progen.com//Antibodies/Research-Area/Cell-Cycle-Proliferation/ cell proliferation], as it prevents cell growth in the absence of a signal. [https://www.ncbi.nlm.nih.gov/books/NBK26877/#:~:text=Extracellular%20Growth%20Factors%20Stimulate%20Cell,Cell%20Growth%2C%20Cell%20Division%2C%20and Extracellular Growth Factors] trigger GTP to bind to MRAS, which triggers SMP formation <ref name="Lavoie">PMID: 35970881</ref>.Upon SMP complex formation, PP1C is brought into close proximity of Ras, leading to the dephosphorylation of Ser259 of Raf by the active site of PP1C <ref name="Lavoie">PMID: 35970881</ref>. Once dephosphorylated, Raf is in the <scene name='95/952695/Autoinhibited_confirmation/8'>active confirmation</scene>, allowing for the interaction of Ras and Raf, and the initiation of the signaling cascade.<ref name="Young">PMID: 30348783</ref> |
== Structure of Subunits == | == Structure of Subunits == | ||
=== SHOC2 === | === SHOC2 === | ||
| - | + | <scene name='95/952695/Shoc2intro/1'>SHOC2</scene> is essential for complex formation. It a crescent shaped complex that serves as a bridge for PP1C and MRAS, maximizing interaction between the three subunits of the SMP complex <ref name="Hauseman">PMID:35830882</ref>. SHOC2 contains a large leucine rich region (LRR) that provides stability and localizes subunit PP1C to the membrane<ref name="Liau">PMID: 35768504</ref>. SHOC2 only undergoes a <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/7'>6° conformational change</scene> when PP1C and MRAS bind, showing it is a scaffolding protein that provides a favorable interface for complex formation<ref name="Liau">PMID: 35768504</ref>. SHOC2 depletion is being studied as a therapeutic approach for RAS-driven cancers due to large scale interactions of the subunits being made possible by SHOC2 <ref name="Kwon">PMID: 35831509</ref>. SHOC2 and PP1C first engage in binding with each other via an N-terminal <scene name='95/952695/Rvxf_motif/2'>RVXF Motif</scene> on SHOC2 that is complimentary to a binding sequence on PP1C. SHOC2 residues <scene name='95/952695/Shoc2_highlighted_residues/1'>V64 and F66</scene> embed in the complimentary region of PP1C, enhancing SHOC2 affinity for PP1C. SHOC2 bind MRAS-GTP through β strands of a LRR that interacts with a hydrophobic region of MRAS-GTP further stabilizing the complex<ref name="Kwon">PMID: 35831509</ref>. | |
=== PP1C === | === PP1C === | ||
| - | <scene name='95/952695/Pp1cintro/3'>The Protein Phosphatase Complex 1 (PP1C)</scene> subunit contains the catalytic site of the SMP complex. The PP1C subunit is a phosphatase enzyme responsible for the removal of a phosphate group on the N-terminal phosphoserine (NTpS) of RAF (Ser259) | + | <scene name='95/952695/Pp1cintro/3'>The Protein Phosphatase Complex 1 (PP1C)</scene> subunit contains the catalytic site of the SMP complex. The PP1C subunit is a phosphatase enzyme responsible for the removal of a phosphate group on the N-terminal phosphoserine (NTpS) of RAF (Ser259)<ref name="Liau">PMID: 35768504</ref>. The exact mechanism of dephosphorylation is currently unknown, but there are three catalytic metal ions: 2 Mn2+ and 1 Cl- present that coordinate nucleophilic water molecules in the active site. This dephosphorylation event allows for pathway activation. Although PP1C can dephosphorylate other proteins independently from the SMP complex, it cannot act on Raf unless bound to the complex because it lacks intrinsic substrate selectivity <ref name="Liau">PMID: 35768504</ref>. SHOC2 and MRAS aid in the specificity of the enzymatic activity. PP1C binds to SHOC2 and MRAS-GTP in a specific orientation that doesn’t change the conformation of the catalytic site and leaves it accessible for substrate binding.''' SHOW IN 2D SURFACE IMAGE''' |
| - | PP1C binds to SHOC2 through a hydrophobic | + | PP1C binds to SHOC2 through a hydrophobic N-terminal disordered region that is complimentary to the <scene name='95/952695/Rvxf_motif/2'>RVXF Motif on SHOC2</scene> and adjacent to the catalytic metal ions. In the Ras/Raf signaling cascade, the region of Raf that is C-terminal to the phosphate group binds to this hydrophobic groove, and the remaining residues bind to the hydrophobic region of SHOC2. Raf binding to this region of SHOC2 is what allows PP1C to be specific when in the SMP complex in comparison to PP1C on its own. PP1C also has a singular cysteine (C291) ''' GREEN LINK'''present in the hydrophobic binding site in order to provide further stability to the substrate-protein interaction by forming a covalent bond to the substrate. Similarly to SHOC2, PP1C does not undergo a significant conformational change when SHOC2 and MRAS-GTP bind. The lack of conformational change shows that the structure of PP1C is not dependent on the SMP complex, but in order to act as a phosphatase it must be bound to the complex <ref name="Liau">PMID: 35768504</ref>. |
PP1C is involved in many different cellular signaling pathways including protein synthesis, muscle contraction, and even carbohydrate metabolism. Wolfgang In all these pathways, including the SMP pathway, PP1C does not exist as a monomer, it is present in holoenzyme form complex with one of two regulatory subunits ensuring there is no sporadic pathway activation. Schulman CITE | PP1C is involved in many different cellular signaling pathways including protein synthesis, muscle contraction, and even carbohydrate metabolism. Wolfgang In all these pathways, including the SMP pathway, PP1C does not exist as a monomer, it is present in holoenzyme form complex with one of two regulatory subunits ensuring there is no sporadic pathway activation. Schulman CITE | ||
| Line 31: | Line 31: | ||
==RAS== | ==RAS== | ||
| - | RAS proteins are GTP-dependent intracellular switches that are anchored to the plasma membrane | + | RAS proteins are GTP-dependent intracellular switches that are anchored to the plasma membrane. <ref name="Liau">PMID: 35768504</ref> RAS proteins activate RAF kinases through direct binding and membrane recruitment, resulting in RAF dimerization and pathway activation <ref name="Liau">PMID: 35768504</ref>. The SMP complex has specificity for MRAS. Other RAS proteins may bind to SHOC2, but MRAS induces the complex formation with a significantly lower Kd (dissociation constant) <ref name="Liau">PMID: 35768504</ref>. There are no known membrane interacting regions on SHOC2 and PP1C, meaning the hydrophobic fatty acid tail on MRAS is responsible for recruiting the complex to the cell membrane <ref name="Hauseman">PMID:35830882</ref>. |
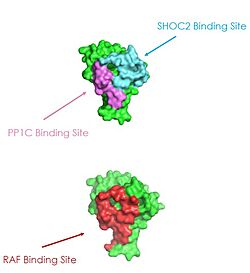
| - | A significant amount of steric overlap is seen in MRAS for the binding sites of PP1C, SHOC2, and Raf. In '''Figure 2''', MRAS is shown in green, with the SHOC2 binding site colored cyan, the PP1C binding site colored green, and the RAF binding site shown in red on a different RAS protein. Hence, multiple RAS proteins are required for further activation of the receptor tyrosine kinase pathway. Due to the significant overlap in binding domains, one MRAS molecule is needed to recruit SHOC2 and PP1C to the membrane, and another RAS molecule is needed activate RAF. The ability of MRAS-GTP to cluster at the cell membrane</scene> is a crucial capability for this protein complex. The presence of this <scene name='95/952695/413cellmemprotrusion/4'>palmitoylated tail</scene> is responsible for this anchoring to the cell membrane, similar to the hydrophobic fatty acid tail on MRAS that is responsible for recruiting SMP to the cell membrane, allowing only for 2D movement and increasing local concentrations of the players needed in this signaling pathway | + | A significant amount of steric overlap is seen in MRAS for the binding sites of PP1C, SHOC2, and Raf. In '''Figure 2''', MRAS is shown in green, with the SHOC2 binding site colored cyan, the PP1C binding site colored green, and the RAF binding site shown in red on a different RAS protein. Hence, multiple RAS proteins are required for further activation of the receptor tyrosine kinase pathway. Due to the significant overlap in binding domains, one MRAS molecule is needed to recruit SHOC2 and PP1C to the membrane, and another RAS molecule is needed activate RAF. The ability of MRAS-GTP to cluster at the cell membrane</scene> is a crucial capability for this protein complex. The presence of this <scene name='95/952695/413cellmemprotrusion/4'>palmitoylated tail</scene> is responsible for this anchoring to the cell membrane, similar to the hydrophobic fatty acid tail on MRAS that is responsible for recruiting SMP to the cell membrane, allowing only for 2D movement and increasing local concentrations of the players needed in this signaling pathway <ref name="Hauseman">PMID:35830882</ref>. |
| - | MRAS contains two regions called Switch I (SWI) and Switch II (SWII) that undergo conformational changes depending if MRAS is bound to GDP or GTP | + | MRAS contains two regions called Switch I (SWI) and Switch II (SWII) that undergo conformational changes depending if MRAS is bound to GDP or GTP <ref name="Liau">PMID: 35768504</ref>. The conformation of these switches determines if the SMP complex can form or not. Mutations to MRAS lead to consistent GTP-loading, causing an increase in the formation of the SMP complex and there is consistent activation of the cell-proliferation pathway in the absence of external growth factors. |
=== Switch I and Switch II === | === Switch I and Switch II === | ||
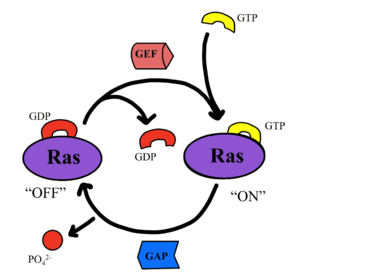
| - | [[Image:GEF2.png|370 px|left|thumb|'''Figure 3''': Nucleotide Exchange Factor | + | [[Image:GEF2.png|370 px|left|thumb|'''Figure 3''': Nucleotide Exchange Factor <ref name="Liau">PMID: 35768504</ref>]]. |
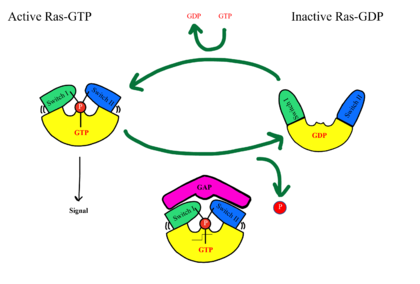
[[Image:RASRAF.png|420 px|right|thumb|'''Figure 4''': MRAS SWI and SWII open and closed conformations.<ref name="Liau">PMID: 35768504</ref>]] | [[Image:RASRAF.png|420 px|right|thumb|'''Figure 4''': MRAS SWI and SWII open and closed conformations.<ref name="Liau">PMID: 35768504</ref>]] | ||
| - | SHOC2-PP1C-MRAS is a central gatekeeper in receptor tyrosine kinase signaling | + | SHOC2-PP1C-MRAS is a central gatekeeper in receptor tyrosine kinase signaling <ref name="Liau">PMID: 35768504</ref>. '''Figure 1''' shows the specific pathways SHOC2-PP1C-MRAS mediates. When MRAS is bound to GDP, shown in the left of '''Figure 1''', Raf is bound to a 14-3-3 protein dimer restricting it to the cytoplasm. When MRAS-GDP is exchanged for GTP via a nucleotide exchange factor GEF, a conformational change occurs. This change, shown in '''Figure 3''', causes a shift from the <scene name='95/952693/Swi_open_conformation/6'>open conformation</scene> to <scene name='95/952693/Switch_i_gtp_bound/11'>closed conformation</scene> of Switch I, shown in '''Figure 4'''. The Switch I (SWI) region is made up of residues 42-48 of the MRAS domain <ref name="Kwon">PMID: 35831509</ref>. These residues are crucial for the binding of MRAS, SHOC2, and PP1C because MRAS undergoes a conformational change that allows for SMP complex assembly upon GTP binding <ref name="Hauseman">PMID:35830882</ref>. When GTP is bound to MRAS, it is in the “closed conformation” because hydrogen bond interactions between the γ phosphate of GTP and residues in the SWI region of MRAS cause SWI to adopt a closed conformation <ref name="Hauseman">PMID:35830882</ref>, as seen in '''Figure 4'''. The closed conformation allows for the binding of SHOC2 and PP1C because there is no steric clash between the <scene name='95/952693/Switch_i_gtp_bound/11'>SWI region of MRAS</scene> and the surface of SHOC2 when GTP is bound <ref name="Kwon">PMID: 35831509</ref>. The only large-scale conformational change occurs in the MRAS subunit <ref name="Liau">PMID: 35768504</ref>. When GDP is bound to the MRAS domain, it is in the “open” conformation. Since the γ-phosphate is not bound to GDP, there are no hydrogen bond interactions with the oxygens of the γ-phosphate group and the MRAS SWI region, causing MRAS to adpot an "open" conformation. Since SHOC2 and PP1C do not undergo much conformational change, they are in a slow equilibrium of binding and unbinding until MRAS binds to GTP allowing MRAS to bind to SHOC2 and PP1C <ref name="Liau">PMID: 35768504</ref>. |
=== Cancer and Rasopathies === | === Cancer and Rasopathies === | ||
Revision as of 00:59, 17 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
SHOC2-PP1C-MRAS
| |||||||||||
Protopedia Resources
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-04928-2. doi:, 10.1038/s41586-022-04928-2. PMID:35831509 doi:http://dx.doi.org/10.1038/s41586-022-04928-2
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Molle S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-05086-1. doi:, 10.1038/s41586-022-05086-1. PMID:35830882 doi:http://dx.doi.org/10.1038/s41586-022-05086-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 4.0 4.1 4.2 4.3 Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. PMID:35970881 doi:10.1038/d41586-022-02189-7
- ↑ 5.0 5.1 Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. PMID:30348783 doi:10.1073/pnas.1720352115
Student Contributors
- Sloan August
- Rosa Trippel
- Kayla Wilhoite