We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1767
From Proteopedia
(Difference between revisions)
| Line 44: | Line 44: | ||
[[Image:RASRAF.png|410 px|right|thumb|'''Figure 5''': MRAS SWI and SWII open and closed conformations<ref name="Liau">PMID: 35768504</ref>.]] | [[Image:RASRAF.png|410 px|right|thumb|'''Figure 5''': MRAS SWI and SWII open and closed conformations<ref name="Liau">PMID: 35768504</ref>.]] | ||
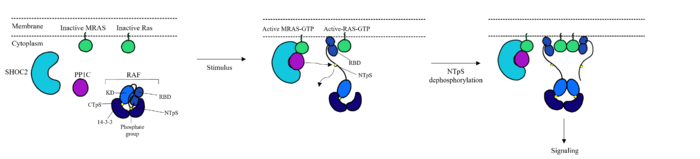
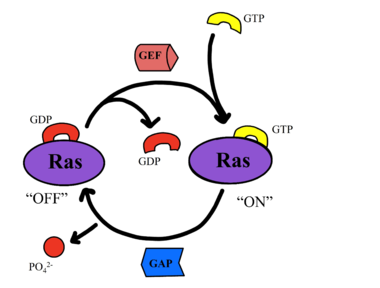
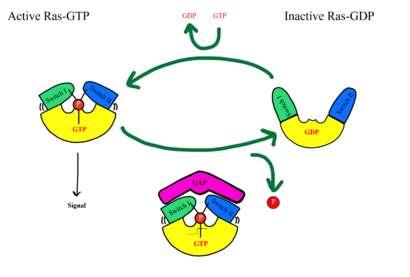
| - | SHOC2-PP1C-MRAS is a central gatekeeper in receptor tyrosine kinase signaling <ref name="Liau">PMID: 35768504</ref>. '''Figure 1''' shows the specific pathways SHOC2-PP1C-MRAS mediates. When MRAS is bound to GDP, shown in the left of '''Figure 1''', RAF is bound to a 14-3-3 protein dimer restricting it to the cytoplasm. When MRAS-GDP is exchanged for GTP via a nucleotide exchange factor GEF, shown in '''Figure 4''', a conformational change occurs. This change', causes a shift from the <scene name='95/952693/Swi_open_conformation/6'>open conformation</scene> to <scene name='95/952693/Switch_i_gtp_bound/11'>closed conformation</scene> of Switch I, shown in '''Figure 5'''. The Switch I (SWI) region is made up of residues 42-48 of the MRAS domain <ref name="Kwon">PMID: 35831509</ref>. These residues are crucial for the binding of MRAS, SHOC2, and PP1C because MRAS undergoes a conformational change that allows for SMP complex assembly upon GTP binding <ref name="Hauseman">PMID:35830882</ref>. When GTP is bound to MRAS, it is in the “closed conformation” because hydrogen bond interactions between the γ phosphate of GTP and residues in the SWI region of MRAS cause SWI to adopt a closed conformation <ref name="Hauseman">PMID:35830882</ref>, as seen in '''Figure 5'''. The closed conformation allows for the binding of SHOC2 and PP1C because there is no [https:// | + | SHOC2-PP1C-MRAS is a central gatekeeper in receptor tyrosine kinase signaling <ref name="Liau">PMID: 35768504</ref>. '''Figure 1''' shows the specific pathways SHOC2-PP1C-MRAS mediates. When MRAS is bound to GDP, shown in the left of '''Figure 1''', RAF is bound to a 14-3-3 protein dimer restricting it to the cytoplasm. When MRAS-GDP is exchanged for GTP via a nucleotide exchange factor GEF, shown in '''Figure 4''', a conformational change occurs. This change', causes a shift from the <scene name='95/952693/Swi_open_conformation/6'>open conformation</scene> to <scene name='95/952693/Switch_i_gtp_bound/11'>closed conformation</scene> of Switch I, shown in '''Figure 5'''. The Switch I (SWI) region is made up of residues 42-48 of the MRAS domain <ref name="Kwon">PMID: 35831509</ref>. These residues are crucial for the binding of MRAS, SHOC2, and PP1C because MRAS undergoes a conformational change that allows for SMP complex assembly upon GTP binding <ref name="Hauseman">PMID:35830882</ref>. When GTP is bound to MRAS, it is in the “closed conformation” because hydrogen bond interactions between the γ phosphate of GTP and residues in the SWI region of MRAS cause SWI to adopt a closed conformation <ref name="Hauseman">PMID:35830882</ref>, as seen in '''Figure 5'''. The closed conformation allows for the binding of SHOC2 and PP1C because there is no [https://pubs.rsc.org/en/content/articlelanding/2020/cp/d0cp03359f. steric clash] between the <scene name='95/952693/Switch_i_gtp_bound/11'>SWI region of MRAS</scene> and the surface of SHOC2 when GTP is bound <ref name="Kwon">PMID: 35831509</ref>. The only large-scale conformational change occurs in the MRAS subunit <ref name="Liau">PMID: 35768504</ref>. When GDP is bound to the MRAS domain, it is in the “open” conformation. Since the γ-phosphate is not bound to GDP, there are no hydrogen bond interactions with the oxygens of the γ-phosphate group and the MRAS SWI region, causing MRAS to adpot an "open" conformation. Since SHOC2 and PP1C do not undergo much conformational change, they are in a slow equilibrium of binding and unbinding until MRAS binds to GTP allowing MRAS to bind to SHOC2 and PP1C <ref name="Liau">PMID: 35768504</ref>. |
=== Cancer and Rasopathies === | === Cancer and Rasopathies === | ||
Revision as of 03:09, 17 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
SHOC2-PP1C-MRAS
| |||||||||||
Protopedia Resources
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-04928-2. doi:, 10.1038/s41586-022-04928-2. PMID:35831509 doi:http://dx.doi.org/10.1038/s41586-022-04928-2
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Molle S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-05086-1. doi:, 10.1038/s41586-022-05086-1. PMID:35830882 doi:http://dx.doi.org/10.1038/s41586-022-05086-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. PMID:35970881 doi:10.1038/d41586-022-02189-7
- ↑ 5.0 5.1 Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. PMID:30348783 doi:10.1073/pnas.1720352115
- ↑ Kelker MS, Page R, Peti W. Crystal structures of protein phosphatase-1 bound to nodularin-R and tautomycin: a novel scaffold for structure-based drug design of serine/threonine phosphatase inhibitors. J Mol Biol. 2009 Jan 9;385(1):11-21. Epub 2008 Nov 1. PMID:18992256 doi:10.1016/j.jmb.2008.10.053
Student Contributors
- Sloan August
- Rosa Trippel
- Kayla Wilhoite