We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1767
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
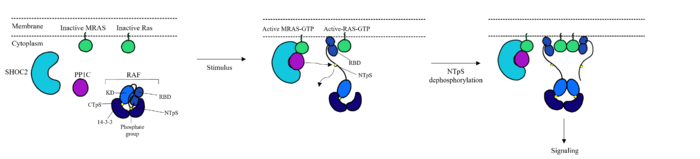
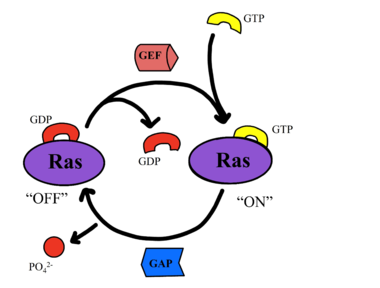
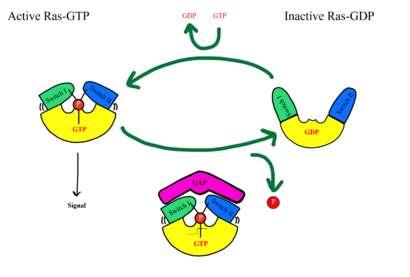
RAS proteins are GTP-dependent [https://pubmed.ncbi.nlm.nih.gov/14604583/. intracellular switches] that are anchored to the plasma membrane. <ref name="Liau">PMID: 35768504</ref> RAS proteins activate RAF kinases through direct binding and membrane recruitment, resulting in RAF dimerization and pathway activation <ref name="Liau">PMID: 35768504</ref>. The SMP complex has specificity for MRAS. Other RAS proteins may bind to SHOC2, but MRAS induces the complex formation with a significantly lower [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6004624/. dissociation constant] <ref name="Liau">PMID: 35768504</ref>. There are no known membrane interacting regions on SHOC2 and PP1C, meaning the [https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/03%3A_Biological_Macromolecules/3.05%3A_Lipid_Molecules_-_Phospholipids#:~:text=The%20fatty%20acid%20chains%20are,the%20intracellular%20and%20extracellular%20fluid. hydrophobic fatty acid tail] on MRAS is responsible for recruiting the complex to the cell membrane allowing only for 2D movement and increasing local concentrations of the players needed in this signaling pathway <ref name="Hauseman">PMID:35830882</ref>. | RAS proteins are GTP-dependent [https://pubmed.ncbi.nlm.nih.gov/14604583/. intracellular switches] that are anchored to the plasma membrane. <ref name="Liau">PMID: 35768504</ref> RAS proteins activate RAF kinases through direct binding and membrane recruitment, resulting in RAF dimerization and pathway activation <ref name="Liau">PMID: 35768504</ref>. The SMP complex has specificity for MRAS. Other RAS proteins may bind to SHOC2, but MRAS induces the complex formation with a significantly lower [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6004624/. dissociation constant] <ref name="Liau">PMID: 35768504</ref>. There are no known membrane interacting regions on SHOC2 and PP1C, meaning the [https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/03%3A_Biological_Macromolecules/3.05%3A_Lipid_Molecules_-_Phospholipids#:~:text=The%20fatty%20acid%20chains%20are,the%20intracellular%20and%20extracellular%20fluid. hydrophobic fatty acid tail] on MRAS is responsible for recruiting the complex to the cell membrane allowing only for 2D movement and increasing local concentrations of the players needed in this signaling pathway <ref name="Hauseman">PMID:35830882</ref>. | ||
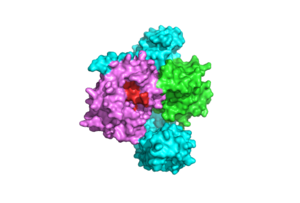
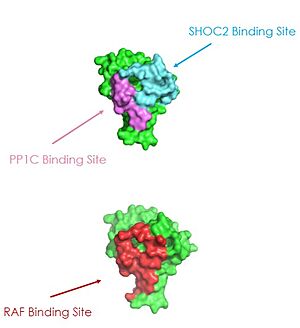
| - | A significant amount of steric overlap is seen in MRAS for the binding sites of PP1C, SHOC2, and RAF <ref name="Liau">PMID: 35768504</ref>. In '''Figure 3''', {{Font color|lime|MRAS}} is shown in green, with the {{Font color|cyan|SHOC2}} binding site colored cyan, the {{Font color| | + | A significant amount of steric overlap is seen in MRAS for the binding sites of PP1C, SHOC2, and RAF <ref name="Liau">PMID: 35768504</ref>. In '''Figure 3''', {{Font color|lime|MRAS}} is shown in green, with the {{Font color|cyan|SHOC2}} binding site colored cyan, the {{Font color|violet|PP1C binding site}} colored violet, and the {{Font color|red|RAF binding site}} shown in red on a different RAS protein. Hence, multiple RAS proteins are required for further activation of the receptor tyrosine kinase pathway <ref name="Lavoie">PMID: 35970881</ref>. Due to the significant overlap in binding domains, one MRAS molecule is needed to recruit SHOC2 and PP1C to the membrane, and another RAS molecule is needed activate RAF <ref name="Lavoie">PMID: 35970881</ref>. The ability of MRAS-GTP to cluster at the cell membrane is a crucial capability for this protein complex. The presence of this <scene name='95/952695/413cellmemprotrusion/4'>palmitoylated tail</scene> is responsible for this anchoring to the cell membrane, similar to the hydrophobic fatty acid tail on MRAS that is responsible for recruiting SMP to the cell membrane. |
Revision as of 03:17, 17 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
SHOC2-PP1C-MRAS
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-04928-2. doi:, 10.1038/s41586-022-04928-2. PMID:35831509 doi:http://dx.doi.org/10.1038/s41586-022-04928-2
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Molle S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-05086-1. doi:, 10.1038/s41586-022-05086-1. PMID:35830882 doi:http://dx.doi.org/10.1038/s41586-022-05086-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. PMID:35970881 doi:10.1038/d41586-022-02189-7
- ↑ 5.0 5.1 Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. PMID:30348783 doi:10.1073/pnas.1720352115
- ↑ Kelker MS, Page R, Peti W. Crystal structures of protein phosphatase-1 bound to nodularin-R and tautomycin: a novel scaffold for structure-based drug design of serine/threonine phosphatase inhibitors. J Mol Biol. 2009 Jan 9;385(1):11-21. Epub 2008 Nov 1. PMID:18992256 doi:10.1016/j.jmb.2008.10.053
Student Contributors
- Sloan August
- Rosa Trippel
- Kayla Wilhoite