We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1792
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
=== Hinge Region=== | === Hinge Region=== | ||
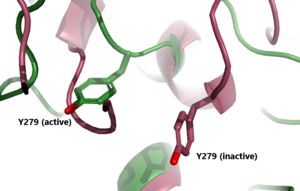
| - | The <scene name='95/952720/Hinge_region_spin/1'>Hinge Region</scene> (purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. Also referred to as the signaling specificity domain the hinge region plays a dual role in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. The hinge region is made up of two α-helices connected via di-sulfide bonds. These two helices help orient TSH properly for LRRD binding. It is proposed that the residues Asp386, Tyr385, and Tyr387 create a negative-charged region on the Helix. This negatively charged region interacts with the positively charged region of TSH created by residue Arg54. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. Conformational changes in this region, specifically the orientation of <scene name='95/952720/Hinge_region_residues/2'>Y279 residue</scene>, are responsible for the bringing TSHR into the active state <ref name="Faust"/> | + | The <scene name='95/952720/Hinge_region_spin/1'>Hinge Region</scene> (purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. Also referred to as the signaling specificity domain the hinge region plays a dual role in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. The hinge region is made up of two α-helices connected via di-sulfide bonds. These two helices help orient TSH properly for LRRD binding. It is proposed that the <scene name='95/952720/Hinge_tsh_residue/1'>Hinge Region residues</scene> Asp386, Tyr385, and Tyr387 create a negative-charged region on the Helix. This negatively charged region interacts with the positively charged region of TSH created by residue Arg54. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. Conformational changes in this region, specifically the orientation of <scene name='95/952720/Hinge_region_residues/2'>Y279 residue</scene>, are responsible for the bringing TSHR into the active state <ref name="Faust"/> |
== Active vs Inactive State== | == Active vs Inactive State== | ||
Revision as of 01:01, 21 April 2023
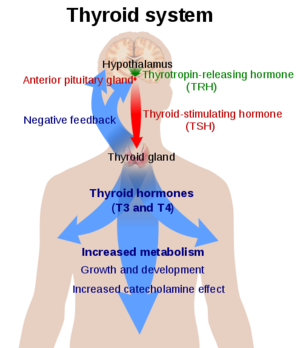
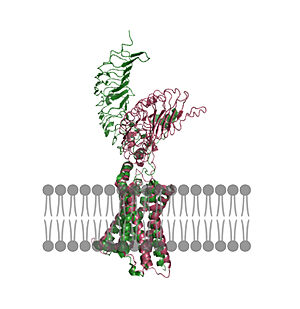
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 3.3 3.4 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ 4.0 4.1 4.2 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 5.0 5.1 Goel R, Raju R, Maharudraiah J, Sameer Kumar GS, Ghosh K, Kumar A, Lakshmi TP, Sharma J, Sharma R, Balakrishnan L, Pan A, Kandasamy K, Christopher R, Krishna V, Mohan SS, Harsha HC, Mathur PP, Pandey A, Keshava Prasad TS. A Signaling Network of Thyroid-Stimulating Hormone. J Proteomics Bioinform. 2011 Oct 29;4:10.4172/jpb.1000195. PMID:24255551 doi:10.4172/jpb.1000195
- ↑ Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]
Student Contributors
- Alex Kem
- Grace Lane