We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Preston Roa/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
===Tirzepatide=== | ===Tirzepatide=== | ||
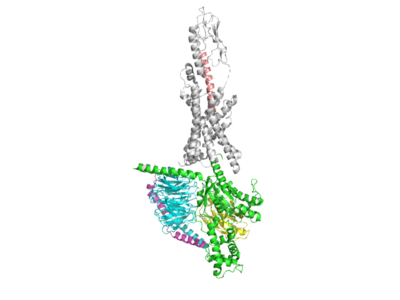
The co-agonist drug, Tirzepatide, is regarded as one of the most promising candidates for better treatment of obesity and type II diabetes by improving weight reduction and glycemic control. Here is a general overview of the Tirzepatide pepetide bound to the GLP-1R <scene name='10/1037513/7rgp_-_tirzepatide_boundcartoo/2'>Tirzepatide Bound Overview Cartoon</scene> <scene name='10/1037513/7rgp_-_tirzepatide_boundsticks/2'>Tirzepatide Bound Overview Sticks</scene>. Tirzepatide is a dual agonist of the glucagon-like-peptide-1 receptor (GLP-1R) and the glucose-dependent insulinotropic polypeptide receptor (GIPR). Co-agonism of both GLP-1 and GIP pathways provides beneficial therapeutic effects by mimicking desirable components of each respective pathway. Tirzepatide exhibits similar pharmacology to the native GIP pathway, but differs with regards to the GLP-1 pathway, as it shows biased signaling of cAMP. This biased signaling, also called differential signaling, allows for the co-agonist drug to increase efficacy in glucose control and body weight regulation for treating type II diabetes as well as obesity. | The co-agonist drug, Tirzepatide, is regarded as one of the most promising candidates for better treatment of obesity and type II diabetes by improving weight reduction and glycemic control. Here is a general overview of the Tirzepatide pepetide bound to the GLP-1R <scene name='10/1037513/7rgp_-_tirzepatide_boundcartoo/2'>Tirzepatide Bound Overview Cartoon</scene> <scene name='10/1037513/7rgp_-_tirzepatide_boundsticks/2'>Tirzepatide Bound Overview Sticks</scene>. Tirzepatide is a dual agonist of the glucagon-like-peptide-1 receptor (GLP-1R) and the glucose-dependent insulinotropic polypeptide receptor (GIPR). Co-agonism of both GLP-1 and GIP pathways provides beneficial therapeutic effects by mimicking desirable components of each respective pathway. Tirzepatide exhibits similar pharmacology to the native GIP pathway, but differs with regards to the GLP-1 pathway, as it shows biased signaling of cAMP. This biased signaling, also called differential signaling, allows for the co-agonist drug to increase efficacy in glucose control and body weight regulation for treating type II diabetes as well as obesity. | ||
| - | One way in which the Tirzepatide peptide fosters biased signaling is by utilizing particular residues from both GLP-1 and GIP in order to carefully stimulate parts of both pathways. One way in which the Tirzepatide peptide differs from both GLP-1 and GIP is seen through two mutations at residue sites 2 and 13. These residues have been mutated to mutant AIB residue which is physiologically vital for the performance of the drug as it prevents cleavage from the enzyme DPP-4, which normally cleaves the native GLP-1 peptide <scene name='10/1037513/7rgp-_2aib_mutation/4'>Tirzepatide 2AIB Mutation</scene> <scene name='10/1037513/7rgp_-_13aib_mutation/4'>Tirzepatide 13AIB Mutation</scene>. Tirzepatide binds similarly to the native GLP-1 peptide to its GLP-1R by forming hydrogen bonds with the receptor. Y1 of the Tirzepatide peptide is seen hydrogen bonding with Q234 of the GLP-1R, allowing for the peptide to be anchored to the receptor <scene name='10/1037513/Y1_hbond_w_q234/8'>Tirz Hbond Y1 Q234</scene>. The Tirzepatide drug is furthermore stabilized to the GLP-1R through pi-stacking interactions between Y10 of the drug and Y145 of the receptor <scene name='10/1037513/Pi_stacking_y10_and_y145/5'>Pi Stacking Y10 (Tirzepatide) and Y145 (GLP-1R)</scene>. | + | One way in which the Tirzepatide peptide fosters biased signaling is by utilizing particular residues from both GLP-1 and GIP in order to carefully stimulate parts of both pathways. |
| + | |||
| + | [[Image:Tirzepatide modifications.jpg]]|100 px|left|thumb|Figure 1. The tirzepatide ligand can be viewed as a combination of both GLP-1 and GIP natural hormones. Shown in red are all the modified residues of tirzepatide. The blue residues are residues that both Tirzepatide and GLP-1 share.]] | ||
| + | |||
| + | One way in which the Tirzepatide peptide differs from both GLP-1 and GIP is seen through two mutations at residue sites 2 and 13. These residues have been mutated to mutant AIB residue which is physiologically vital for the performance of the drug as it prevents cleavage from the enzyme DPP-4, which normally cleaves the native GLP-1 peptide <scene name='10/1037513/7rgp-_2aib_mutation/4'>Tirzepatide 2AIB Mutation</scene> <scene name='10/1037513/7rgp_-_13aib_mutation/4'>Tirzepatide 13AIB Mutation</scene>. Tirzepatide binds similarly to the native GLP-1 peptide to its GLP-1R by forming hydrogen bonds with the receptor. Y1 of the Tirzepatide peptide is seen hydrogen bonding with Q234 of the GLP-1R, allowing for the peptide to be anchored to the receptor <scene name='10/1037513/Y1_hbond_w_q234/8'>Tirz Hbond Y1 Q234</scene>. The Tirzepatide drug is furthermore stabilized to the GLP-1R through pi-stacking interactions between Y10 of the drug and Y145 of the receptor <scene name='10/1037513/Pi_stacking_y10_and_y145/5'>Pi Stacking Y10 (Tirzepatide) and Y145 (GLP-1R)</scene>. | ||
Additionally, there are certain aspects of the Tirzepatide peptide design which favor the GIP pathway over the GLP-1 pathway. This area of biased signalling is seen through steric clashing between the Tirzepatide peptide and the GLP-1R, which was not seen with the native GLP-1 bound to GLP-1R | Additionally, there are certain aspects of the Tirzepatide peptide design which favor the GIP pathway over the GLP-1 pathway. This area of biased signalling is seen through steric clashing between the Tirzepatide peptide and the GLP-1R, which was not seen with the native GLP-1 bound to GLP-1R | ||
<scene name='10/1037513/Tirz_intxn_glp-1r_steric_clash/3'> Tirzepatide bound to GLP-1R steric clashing</scene> <scene name='10/1037513/Glp_bound_to_glp-1r_no_clash/3'>GLP-1 bound to GLP-1R: interactions, no steric clash</scene>. The lack of steric clashing in the native interaction suggests that this area of the Tirzepatide peptide is important for biased GIP pathway stimulation as an interaction which was found to increase stability of the native GLP-1 bound to GLP-1R is lost with regards to the co-agonist drug Tirzepatide. | <scene name='10/1037513/Tirz_intxn_glp-1r_steric_clash/3'> Tirzepatide bound to GLP-1R steric clashing</scene> <scene name='10/1037513/Glp_bound_to_glp-1r_no_clash/3'>GLP-1 bound to GLP-1R: interactions, no steric clash</scene>. The lack of steric clashing in the native interaction suggests that this area of the Tirzepatide peptide is important for biased GIP pathway stimulation as an interaction which was found to increase stability of the native GLP-1 bound to GLP-1R is lost with regards to the co-agonist drug Tirzepatide. | ||
Revision as of 03:04, 30 April 2024
GLP-1
| |||||||||||
References
[1] Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017 Dec 1;127(12):4217-4227. doi: 10.1172/JCI97233. Epub 2017 Dec 1. PMID: 29202475; PMCID: PMC5707151.
[2] Mayendraraj, A., Rosenkilde, M. M., & Gasbjerg, L. S. (2022). GLP-1 and GIP receptor signaling in beta cells - A review of receptor interactions and co-stimulation. Peptides, 151, 170749. https://doi.org/10.1016/j.peptides.2022.170749
[3] Seino, Y., Fukushima, M., & Yabe, D. (2010). GIP and GLP-1, the two incretin hormones: Similarities and differences. Journal of diabetes investigation, 1(1-2), 8–23. https://doi.org/10.1111/j.2040-1124.2010.00022.x
Student Contributors
- Preston Roa
- Jack Guckien
- Sam Reichenbach