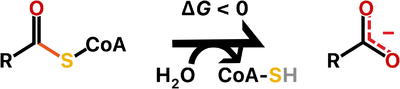Mitochondrial hotdog-fold thioesterase
From Proteopedia
| Line 22: | Line 22: | ||
Notwithstanding that hotdog-fold thioesterases are mainly grouped by their atomic structure since there is little similarity in their primary structure, it is notable that Them4 possesses a conserved <scene name='10/1049462/Hggdt/4'>HGG…D…T</scene> motif also observed in [https://en.wikipedia.org/wiki/Sequence_homology#Orthology orthologs] and its paralogs. | Notwithstanding that hotdog-fold thioesterases are mainly grouped by their atomic structure since there is little similarity in their primary structure, it is notable that Them4 possesses a conserved <scene name='10/1049462/Hggdt/4'>HGG…D…T</scene> motif also observed in [https://en.wikipedia.org/wiki/Sequence_homology#Orthology orthologs] and its paralogs. | ||
In Them4, the catalytic residues are <scene name='10/1049462/Thr-asp/4'>Asp161 and Thr177</scene>, which <scene name='10/1049462/H-bond_activesite/1'>interact</scene> through a [[hydrogen bond]] between the carboxylate in aspartate and the [https://en.wikipedia.org/wiki/Hydroxy_group hydroxyl] in threonine. | In Them4, the catalytic residues are <scene name='10/1049462/Thr-asp/4'>Asp161 and Thr177</scene>, which <scene name='10/1049462/H-bond_activesite/1'>interact</scene> through a [[hydrogen bond]] between the carboxylate in aspartate and the [https://en.wikipedia.org/wiki/Hydroxy_group hydroxyl] in threonine. | ||
| - | In the proposed [https://en.wikipedia.org/wiki/Enzyme_catalysis catalytic mechanism], the deprotonated aspartate residue abstracts a proton from a water molecule, making it very '''reactive''' and prone to a [https://en.wikipedia.org/wiki/Nucleophile nucleophilic attack] on the thioester bond. | + | In the proposed [https://en.wikipedia.org/wiki/Enzyme_catalysis catalytic mechanism], '''the deprotonated aspartate residue abstracts a proton from a water molecule''', making it very '''reactive''' and prone to a [https://en.wikipedia.org/wiki/Nucleophile nucleophilic attack] on the thioester bond. |
As observed in other single hotdog-fold thioesterases, the [[biological assembly]] Them4 is a [https://en.wikipedia.org/wiki/Protein_dimer homodimer] with a '''2-fold symmetry axis'''. This <scene name='10/1049462/Dimer-hotdog/1'>dimer</scene> is maintained mainly by a '''network of hydrogen bonds''' between the residues from the <scene name='10/1049462/Strand6/5'>6th strand</scene> in each monomer. Notably, this network involves the backbone in strand 6 between the beta sheets as well as the side chain of <scene name='10/1049462/Strand6-atoms/2'>Asn179, Asn181 and Asn183</scene> from the same strand. As a result, the homodimer has a <scene name='10/1049462/Betasheet-dimer/2'>continuous antiparallel 12-stranded beta sheet</scene> around <scene name='10/1049462/Hotdog_dimer/1'>two central alpha helixes</scene> oriented antiparallel to one another. | As observed in other single hotdog-fold thioesterases, the [[biological assembly]] Them4 is a [https://en.wikipedia.org/wiki/Protein_dimer homodimer] with a '''2-fold symmetry axis'''. This <scene name='10/1049462/Dimer-hotdog/1'>dimer</scene> is maintained mainly by a '''network of hydrogen bonds''' between the residues from the <scene name='10/1049462/Strand6/5'>6th strand</scene> in each monomer. Notably, this network involves the backbone in strand 6 between the beta sheets as well as the side chain of <scene name='10/1049462/Strand6-atoms/2'>Asn179, Asn181 and Asn183</scene> from the same strand. As a result, the homodimer has a <scene name='10/1049462/Betasheet-dimer/2'>continuous antiparallel 12-stranded beta sheet</scene> around <scene name='10/1049462/Hotdog_dimer/1'>two central alpha helixes</scene> oriented antiparallel to one another. | ||
Additionally, there are other interactions that contribute in stabilizing the homodimer. As an example from Them4, there is a <scene name='10/1049462/Phe_cluster/2'>cluster of phenylalanine side chains</scene> - involving Phe111, Phe115 and Phe121 from both subunits - in another interface region which is stabilized by [https://en.wikipedia.org/wiki/Hydrophobic_effect#Folding_of_macromolecules hydrophobic effect]. | Additionally, there are other interactions that contribute in stabilizing the homodimer. As an example from Them4, there is a <scene name='10/1049462/Phe_cluster/2'>cluster of phenylalanine side chains</scene> - involving Phe111, Phe115 and Phe121 from both subunits - in another interface region which is stabilized by [https://en.wikipedia.org/wiki/Hydrophobic_effect#Folding_of_macromolecules hydrophobic effect]. | ||
| - | In this quaternary structure, for Them4 the catalytic residues from one monomer are <scene name='10/1049462/Dimer-hotdog-hggdt/5'>in proximity</scene> to His152, Gly153 and Gly154 from the other monomer, which are proposed to accommodate the thioester substrate within the active site. | + | In this quaternary structure, for Them4 the catalytic residues from one monomer are <scene name='10/1049462/Dimer-hotdog-hggdt/5'>in proximity</scene> to His152, Gly153 and Gly154 from the other monomer, which are proposed to accommodate the thioester [https://en.wikipedia.org/wiki/Substrate_(chemistry) substrate] within the active site. |
As a direct consequence, in each catalytically competent Them4 there are <scene name='10/1049462/Dimer-hotdog-activesites/2'>two active sites</scene> located in the interface between monomers of the obligatory homodimer. | As a direct consequence, in each catalytically competent Them4 there are <scene name='10/1049462/Dimer-hotdog-activesites/2'>two active sites</scene> located in the interface between monomers of the obligatory homodimer. | ||
Revision as of 21:50, 1 June 2024
Overview of thioesterases
Thioesterases are enzymes that catalyze the hydrolysis of thioester bonds, which are the linkage between a carbonyl and a sulfur atom.
The ATP-dependent formation of a thioester bond from a carboxylate and a thiol in biomolecules makes them more reactive and is particularly an important commitment step in lipid metabolism. Therefore, thioesterases counteract this activation by releasing upon hydrolysis a molecule with the more stable carboxylate group. For this reason, thioesterases are found at the end of some metabolic pathways but they also may act as regulators of flux. Besides lipid metabolism, thioester bonds also occur in biosynthetic pathways for polyketide and non-ribosomal peptide production, as well as in main metabolites of carbon metabolism such as acetyl-CoA and succinyl-CoA.
There are two main families of thioesterases which are distinguished by their folding, named the α/β-hydrolases and the hotdog-fold hydrolases. Notably, these two different families are evolutionarily distant, so the thioesterase activity is a shared feature owing to convergent evolution.
| |||||||||||
Function
From enzymatic activities in vitro, it was shown that Them4 (Zhao et al., 2009) and Them5 (Zhuravleva et al., 2012) have higher kcat/KM for acyl-CoA's with medium and long hydrocarbon chain, such as myristoyl-CoA (14:0), palmitoyl-CoA (16:0), oleoyl-CoA (18:1) and linoleoyl-CoA (18:2). According to Zhuravleva et al. (2012), linoleoyl-CoA (18:2) was a preferred substrate for Them5. From studies with Them5−/− mice, it was identified by mass spectrometry (MS) that loss of Them5 is related to an increase in the levels of monolysocardiolipin (MLCL), which is a metabolite upstream of the cardiolipin remodeling process in mitochondria. Furthermore, the lipidomics analysis by MS for Them5−/− mice also revealed a 2-fold decrease of free fatty acids, notably linoleic (18:2) and linolenic (18:3) acids. This is consistent with the in vitro assay for the recombinant ∆34Them5 which revealed higher kcat/KM for linoleoyl-CoA (18:2). Moreover, it is observed by two-dimensional electron microscopy (2D-EM) and subsequent 3D reconstruction that in hepatocytes from Them5−/− mice, mitochondria were more elongated and interconnected, with a 2-fold increase in volume. With these data, Zhuravleva et al. (2012) propose that Them5 might be a regulator of cardiolipin remodeling through modulation of the unsaturated acyl-CoA pool in mitochondria. This modulation in turn seems to affect mitochondrial morphology.
Zhao et al. (2012) observed that Them4 shows very weak binding affinity (Ki > 1 mM) for carboxylic acids generated after the thioester bond hydrolysis, suggesting that this enzyme is not regulated by product inhibition.
Interestingly, it was revealed that Them2 interacts physically with the protein StarD2/PC-TP, which has a START (StAR-related lipid-transfer, with StAR standing for Steroidogenic Acute Regulatory protein) domain. More specifically, Kanno et al. (2007) observed that StarD2 stimulates myristoyl-CoA hydrolysis catalyzed by Them2. It becomes even more curious when considering that Them1/ACOT11, another mammalian hotdog-fold thioesterase, has in its sequence an additional START domain.
Them4 is also called Akt Carboxyl-Terminal Modulator Protein (CTMP), owing to previous data suggesting that it interacts with the serine-threonine protein kinase Akt1 in an inferred mechanism of regulating apoptosis. At the plasma membrane, CTMP inhibits Akt by preventing its phosphorylation on key residues, threonine 308 and serine 473. This inhibition by CTMP reduces Akt's activity, which is crucial in insulin signaling, cellular survival, and transformation (Maira et al., 2001). However, this activity is not well defined yet. Through pull-down assays, Zhao et al. (2012) verified that Them4 and Akt1 form a stable complex and that Them4 inhibits Akt1 activity in vitro, but Akt1 does not inhibit Them4. In pathological contexts, such as skeletal muscle atrophy, CTMP has been shown to exacerbate muscle degeneration by reducing Akt signaling, thereby increasing muscle catabolism and decreasing protein synthesis (Wang et al., 2023). In glioblastomas, CTMP expression is often downregulated due to promoter hypermethylation, removing its inhibitory effects on Akt and contributing to tumorigenesis (Knobbe et al., 2004). Conversely, in head and neck squamous cell carcinoma (HNSCC), elevated CTMP levels enhance Akt phosphorylation, promoting tumor growth and metastasis, and correlate with poor prognosis (Chang et al., 2016).
References
Caswell, B. T., de Carvalho, C. C., Nguyen, H., Roy, M., Nguyen, T., & Cantu, D. C. (2022). Thioesterase enzyme families: Functions, structures, and mechanisms. Protein Science, 31(3), 652-676. https://doi.org/10.1002/pro.4263
Chang, J. W., Jung, S.-N., Kim, J.-H., Shim, G.-A., Hee Sung Park, Liu, L., Jin Man Kim, Park, J., & Bon Seok Koo. (2016). Carboxyl-Terminal Modulator Protein Positively Acts as an Oncogenic Driver in Head and Neck Squamous Cell Carcinoma via Regulating Akt phosphorylation. Scientific Reports, 6(1). https://doi.org/10.1038/srep28503
Knobbe, C. B., Reifenberger, J., Blaschke, B., & Reifenberger, G. (2004). Hypermethylation and Transcriptional Downregulation of the Carboxyl-Terminal Modulator Protein Gene in Glioblastomas. Journal of the National Cancer Institute, 96(6), 483–486. https://doi.org/10.1093/jnci/djh064
Maira, S.-M., Galetic, I., Brazil, D. P., Kaech, S., Ingley, E., Thelen, M., & Hemmings, B. A. (2001). Carboxyl-Terminal Modulator Protein (CTMP), a Negative Regulator of PKB/Akt and v-Akt at the Plasma Membrane. Science, 294(5541), 374–380. https://doi.org/10.1126/science.1062030
Swarbrick, C. M., Nanson, J. D., Patterson, E. I., & Forwood, J. K. (2020). Structure, function, and regulation of thioesterases. Progress in Lipid Research, 79, 101036. https://doi.org/10.1016/j.plipres.2020.101036
Cheng, Z., Song, F., Shan, X., Wei, Z., Wang, Y., Dunaway-Mariano, D., & Gong, W. (2006). Crystal structure of human thioesterase superfamily member 2. Biochemical and biophysical research communications, 349(1), 172-177. https://doi.org/10.1016/j.bbrc.2006.08.025
Wei, J., Kang, H. W., & Cohen, D. E. (2009). Thioesterase superfamily member 2 (Them2)/acyl-CoA thioesterase 13 (Acot13): a homotetrameric hotdog fold thioesterase with selectivity for long-chain fatty acyl-CoAs. Biochemical Journal, 421(2), 311-322. https://doi.org/10.1042/BJ20090039
Kanno, K., Wu, M. K., Agate, D. S., Fanelli, B. J., Wagle, N., Scapa, E. F., ... & Cohen, D. E. (2007). Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2. Journal of Biological Chemistry, 282(42), 30728-30736. https://doi.org/10.1074/jbc.M703745200
Kunishima, N., Asada, Y., Sugahara, M., Ishijima, J., Nodake, Y., Sugahara, M., ... & Sugahara, M. (2005). A novel induced-fit reaction mechanism of asymmetric hot dog thioesterase PAAI. Journal of molecular biology, 352(1), 212-228. https://doi.org/10.1016/j.jmb.2005.07.008
Cantu, D. C., Ardèvol, A., Rovira, C., & Reilly, P. J. (2014). Molecular mechanism of a hotdog‐fold acyl‐CoA thioesterase. Chemistry–A European Journal, 20(29), 9045-9051. https://doi.org/10.1002/chem.201304228
Wang, J., Tierney, L., Wilson, C., Phillips, V., Goldman, L., Mumaw, C., En Muang, & Walker, C. L. (2023). Carboxyl-terminal modulator protein (CTMP) deficiency mitigates denervation-induced skeletal muscle atrophy. Biochemical and Biophysical Research Communications, 644, 155–161. https://doi.org/10.1016/j.bbrc.2023.01.023
Zhao, H., Martin, B. M., Bisoffi, M., & Dunaway-Mariano, D. (2009). The Akt C-terminal modulator protein is an acyl-CoA thioesterase of the Hotdog-Fold family. Biochemistry, 48(24), 5507-5509. https://doi.org/10.1021/bi900710w
Zhao, H., Lim, K., Choudry, A., Latham, J. A., Pathak, M. C., Dominguez, D., ... & Dunaway-Mariano, D. (2012). Correlation of structure and function in the human hotdog-fold enzyme hTHEM4. Biochemistry, 51(33), 6490-6492. https://doi.org/10.1021/bi300968n
Zhuravleva, E., Gut, H., Hynx, D., Marcellin, D., Bleck, C. K., Genoud, C., ... & Hemmings, B. A. (2012). Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development. Molecular and cellular biology, 32(14), 2685-2697. https://doi.org/10.1128/MCB.00312-12
Proteopedia Page Contributors and Editors (what is this?)
Marcelo Mesa, Thabata Fernanda Oliveira, Eduardo Ferraro, Michal Harel