Synthetic nanomaterials from standardized protein blocks
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
In this project, the simplest building blocks consist of anti-parallel alpha helices engineered to be straight and flat, that is ''twistless helix repeat'' (THR) protein blocks. A simple example, THR1, is [[8g9j]], consisting of <scene name='10/1068508/8g9j_flat_square/3'>eight anti-parallel alpha helices with seven turns per helix</scene><ref>An amino-terminal histidine tag was not resolved in the electron density map of [[8g9j]], and thus is missing in the structure depicted.</ref>. Each helix is amphipathic, that is, hydrophobic on the side contacting other helixes, and hydrophilic on the side facing outwards (not shown). The 2.5 Å [[resolution]] of [[8g9j]] enabled the modeling of all helix side chains. Non-covalent interactions between helices are nearly all apolar, with a few hydrogen bonds, and two salt bridges (not shown). The <scene name='10/1068508/8g9j_flat_square/4'>block surface is designed to have many charges</scene>, making a highly water soluble building block. The edges of the block are "capped" with charges that prevent these blocks from binding to each other, thus enabling crystallization of this block rather than having it precipitate. | In this project, the simplest building blocks consist of anti-parallel alpha helices engineered to be straight and flat, that is ''twistless helix repeat'' (THR) protein blocks. A simple example, THR1, is [[8g9j]], consisting of <scene name='10/1068508/8g9j_flat_square/3'>eight anti-parallel alpha helices with seven turns per helix</scene><ref>An amino-terminal histidine tag was not resolved in the electron density map of [[8g9j]], and thus is missing in the structure depicted.</ref>. Each helix is amphipathic, that is, hydrophobic on the side contacting other helixes, and hydrophilic on the side facing outwards (not shown). The 2.5 Å [[resolution]] of [[8g9j]] enabled the modeling of all helix side chains. Non-covalent interactions between helices are nearly all apolar, with a few hydrogen bonds, and two salt bridges (not shown). The <scene name='10/1068508/8g9j_flat_square/4'>block surface is designed to have many charges</scene>, making a highly water soluble building block. The edges of the block are "capped" with charges that prevent these blocks from binding to each other, thus enabling crystallization of this block rather than having it precipitate. | ||
| - | The sequences and binding interfaces of building blocks were designed using Rosetta FastDesign and mainly ProteinMPNN<ref>PMID: 38502697</ref><ref>PMID: 38194293</ref><ref>PMID: 36108050</ref>. Designed sequences were filtered according to likelihood of desired folding and assembly as predicted by [[AlphaFold]]2. | + | The '''sequences and binding interfaces''' of building blocks were designed using Rosetta FastDesign and mainly '''ProteinMPNN'''<ref>PMID: 38502697</ref><ref>PMID: 38194293</ref><ref>PMID: 36108050</ref>. Designed sequences were filtered according to likelihood of desired folding and assembly as predicted by [[AlphaFold]]2. |
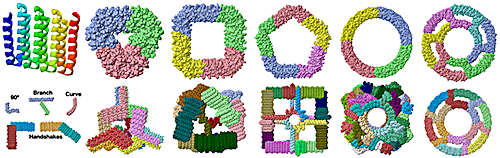
===Five examples of building blocks=== | ===Five examples of building blocks=== | ||
Revision as of 18:59, 5 January 2025
This page is under construction. This notice will be removed when it is ready. Eric Martz 21:20, 23 December 2024 (UTC) |
| |||||||||||
See Also
- Related work from the Baker group includes Bond-centric modular design of protein assemblies by Wang et al., 2024[8].
- Metal-Ligand Polyhedra
References
- ↑ 1.0 1.1 1.2 1.3 Huddy TF, Hsia Y, Kibler RD, Xu J, Bethel N, Nagarajan D, Redler R, Leung PJY, Weidle C, Courbet A, Yang EC, Bera AK, Coudray N, Calise SJ, Davila-Hernandez FA, Han HL, Carr KD, Li Z, McHugh R, Reggiano G, Kang A, Sankaran B, Dickinson MS, Coventry B, Brunette TJ, Liu Y, Dauparas J, Borst AJ, Ekiert D, Kollman JM, Bhabha G, Baker D. Blueprinting extendable nanomaterials with standardized protein blocks. Nature. 2024 Mar;627(8005):898-904. PMID:38480887 doi:10.1038/s41586-024-07188-4
- ↑ An amino-terminal histidine tag was not resolved in the electron density map of 8g9j, and thus is missing in the structure depicted.
- ↑ de Haas RJ, Brunette N, Goodson A, Dauparas J, Yi SY, Yang EC, Dowling Q, Nguyen H, Kang A, Bera AK, Sankaran B, de Vries R, Baker D, King NP. Rapid and automated design of two-component protein nanomaterials using ProteinMPNN. Proc Natl Acad Sci U S A. 2024 Mar 26;121(13):e2314646121. PMID:38502697 doi:10.1073/pnas.2314646121
- ↑ Sumida KH, Núñez-Franco R, Kalvet I, Pellock SJ, Wicky BIM, Milles LF, Dauparas J, Wang J, Kipnis Y, Jameson N, Kang A, De La Cruz J, Sankaran B, Bera AK, Jiménez-Osés G, Baker D. Improving Protein Expression, Stability, and Function with ProteinMPNN. J Am Chem Soc. 2024 Jan 9. PMID:38194293 doi:10.1021/jacs.3c10941
- ↑ Dauparas J, Anishchenko I, Bennett N, Bai H, Ragotte RJ, Milles LF, Wicky BIM, Courbet A, de Haas RJ, Bethel N, Leung PJY, Huddy TF, Pellock S, Tischer D, Chan F, Koepnick B, Nguyen H, Kang A, Sankaran B, Bera AK, King NP, Baker D. Robust deep learning-based protein sequence design using ProteinMPNN. Science. 2022 Sep 15:eadd2187. doi: 10.1126/science.add2187. PMID:36108050 doi:http://dx.doi.org/10.1126/science.add2187
- ↑ The five modules shown are from the following assemblies that can be downloaded as PDB files from supplementary materials of Huddy et al,, 2024: 90° from strut_C6_16. Branch from TT_rail+_tie+. Curve from R20A. Handshake 90° from cage_O4_32. Handshake obtuse angle from cage_I3_8.
- ↑ The assemblies shown can be downloaded as PDB files from supplementary materials of Huddy et al,, 2024.
- ↑ Wang S, Favor A, Kibler R, Lubner J, Borst AJ, Coudray N, Redler RL, Chiang HT, Sheffler W, Hsia Y, Li Z, Ekiert DC, Bhabha G, Pozzo LD, Baker D. Bond-centric modular design of protein assemblies. bioRxiv [Preprint]. 2024 Oct 12:2024.10.11.617872. PMID:39416012 doi:10.1101/2024.10.11.617872