User:Anjali Rabindran/Sandbox 1
From Proteopedia
| Line 49: | Line 49: | ||
The collapse of the tetrahedral intermediate reforms the double bond at the carbonyl, while the catalytic histidine (H242) protonates the leaving group monomer of the PET substrate. H242 then activates a water molecule as a nucleophile to attack the intermediate at S165. | The collapse of the tetrahedral intermediate reforms the double bond at the carbonyl, while the catalytic histidine (H242) protonates the leaving group monomer of the PET substrate. H242 then activates a water molecule as a nucleophile to attack the intermediate at S165. | ||
| - | <br> <center>[[Image: | + | <br> <center>[[Image:Part_CFinal.jpg|500 px|center|thumb|Figure 3. Final step of PET hydrolysis by PETase.]]</center> |
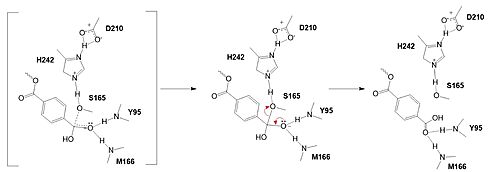
In the final step, the water-activated nucleophile attacks the carbonyl carbon of S165, generating a second tetrahedral intermediate. The collapse of this intermediate regenerates the carbonyl double bond and removes the catalytic serine residue. The oxyanion hole residues stabilize the negative charge of this transition state, allowing for product release of the PET monomer. | In the final step, the water-activated nucleophile attacks the carbonyl carbon of S165, generating a second tetrahedral intermediate. The collapse of this intermediate regenerates the carbonyl double bond and removes the catalytic serine residue. The oxyanion hole residues stabilize the negative charge of this transition state, allowing for product release of the PET monomer. | ||
Revision as of 13:49, 15 April 2025
Contents |
Mutations
|
Researchers have investigated various PET hydrolase mutations to enhance its catalytic ability. One group of researchers, Tournier et. al., have made mutations in the PET hydrolase active site. They identified the key residues involved in the catalytic mechanism by using a model of the (2-HE(MHET)₃) onto the enzyme (PDB ID 4EB0). The site, mainly a hydrophobic pocket, contained 11 residues targeted for mutagenesis. From this, they identified that the majority of enzymes' specific activity went down; however, the mutation of the to either isoleucine or tryptophan increased specific activity.
The mutation of the F243 position to a tryptophan () was selected for further analysis based on its enhanced catalytic activity in the depolymerization of Pf-PET. The W243 mutation improved the substrate's binding affinity and increased the enzyme’s activity compared to the wild-type enzyme. This was one of the few variants that exhibited higher activity, and it was further analyzed through differential scanning fluorimetry (DSF) to assess its stability.[1]
Similar to the W243 mutation, the mutation was identified as a variant with improved depolymerization activity of Pf-PET. The I243 mutation in LCC led to better substrate interaction than the wild-type enzyme. After generating all possible variants, this mutation was among the few that exhibited 75% or more of the wild-type specific activity. As with the W243 mutation, DSF was used to determine the melting temperature and thermal stability, supporting the increased activity observed with this mutation.
Results
Table 1. Specific activity and rate of wild-type, WCCG, and ICCG mutants. Initial rate was measured by the calculated rate of reaction by NaOH consumption. Specific activity was measured via pf-PET-depolymerization assay.[1]
| Enzyme | Initial rate (ghydrolyzed PET•L-1•h-1) | Specific activity (mgTAeqh-1•mgenzyme-1) ± SD | |||
|---|---|---|---|---|---|
| Wild-Type | 25.7 | 81.9 ± 5.6 | |||
| WCCG | 30.3 | 75.9 ± 5.9 | |||
| ICCG | 31.0 | 82.0 ± 3.9 |
Both the WCCG and ICCG mutants display slightly higher initial rates (30.3 and 31.0 g hydrolyzed PET•L⁻¹•h⁻¹, respectively) compared to the wild-type enzyme (25.7 g hydrolyzed PET•L⁻¹•h⁻¹). This suggests that the mutations introduced in WCCG and ICCG enhance the rate of PET breakdown.
The specific activity of the WCCG mutant (75.9 mg TAeq h⁻¹ mg⁻¹ enzyme) is slightly lower than that of wild-type PETase (81.9 mg TAeq h⁻¹ mg⁻¹ enzyme), whereas the ICCG mutant shows comparable specific activity to the wild-type (82.0 mg TAeq h⁻¹ mg⁻¹ enzyme).
Mechanism
Polyethylene terephthalate (PET) hydrolase (PETase) is a serine hydrolase that catalyzes the cleavage of ester bonds in PET polymers.
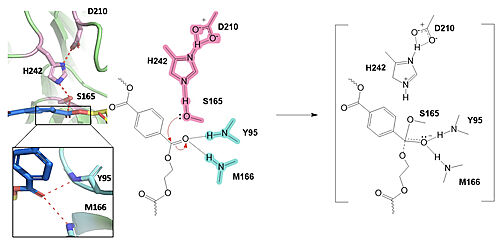
In the first step of the reaction, the nucleophilic serine residue (S165) attacks the carbonyl carbon of the PET substrate, forming a tetrahedral intermediate. The oxyanion hole stabilizes the negative charge.
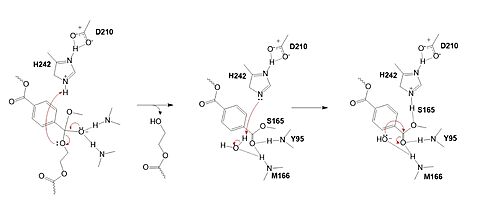
The collapse of the tetrahedral intermediate reforms the double bond at the carbonyl, while the catalytic histidine (H242) protonates the leaving group monomer of the PET substrate. H242 then activates a water molecule as a nucleophile to attack the intermediate at S165.
In the final step, the water-activated nucleophile attacks the carbonyl carbon of S165, generating a second tetrahedral intermediate. The collapse of this intermediate regenerates the carbonyl double bond and removes the catalytic serine residue. The oxyanion hole residues stabilize the negative charge of this transition state, allowing for product release of the PET monomer.
References
- ↑ 1.0 1.1 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4