Sand box 326
From Proteopedia
| Line 15: | Line 15: | ||
== Family and Superfamily == | == Family and Superfamily == | ||
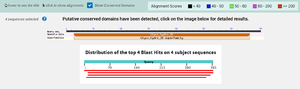
| - | Research | + | Research suggests that 3CBW is a member of the Glycoside Hydrolase super family which was found using BLAST. This means that the protein possibly has the ability to hydrolyze glycosidic bonds between two or more carbohydrates. Additionally, 3CBW is also recognized under the beta-mannanase family. This was confirmed with InterPro which showed 3CBW being highly conserved with this family, having over 300 shared amino acids. |
| - | + | Using Dali, it was found that 3CBW was similar in structure and sequence to proteins 2QHA (Beta-1,4-mannanase) and 2WHK (Mannan endo-1,4-beta-mannosidase) which come from the bacterial species Bacillus Subtilis. Furthermore, BLAST confirmed that 3CBW is similar to the enzymes that come from this bacterial species in terms of functionality. | |
| + | |||
| + | Through a search on InterPro, it was determined that 3CBW has a glycosyl hydrolase family 26 domain. This domain consists mostly of endo-beta-1,4-mannanases and typically has a beta/alpha 8-barrel folding motif. Proteins in this domain also tend to have two Glu residues located respectfully on strands beta-4 and beta-7 that act as the catalytic acid/base and nucleophile in a double-displacement mechanism. | ||
| - | Regarding what protein family 4Q7Q belongs to, DALI results suggest it is a part of a sub-family of the greater GDSL/SGNH superfamily. A PDB90% DALI search labels 4Q7Q as a part of the “Lipolytic Protein G-D-S-L Family,” which refers to enzymes that hydrolyze lipid substrates. | ||
== Sequence Analysis (DONE) == | == Sequence Analysis (DONE) == | ||
Revision as of 11:59, 28 April 2025
3CBW Structure and Proposed Functionality
(NOTE TO ALL EDITORS: This page is part of a final project for a biochemistry lab at Elizabethtown College. Please do not edit this.)
3CBW is a homodimeric protein complex that originates from the bacterial species Chitinophaga Pinensis and has a mass of 80.65 kDa. It is a member of the SGNH Hydrolase Superfamily with structural and sequential similarities to esterases and lipases. Current evidence suggests it causes the hydrolysis of esters and/or acetyl groups on lipids/lipid-like molecules via a serine protease-like active site.
| |||||||||||
References (DONE)
A) Dhawan, S.; Kaur, J.; Microbial Mannanases: An Overview of Production and Applications. Critical Reviews in Biotechnology 2007, 27, 197-216. DOI: 10.1080/07388550701775919 B) Soni, H.; Rawat, H. K.; Pletschke, B. I.; Kango, N. Purification and characterization of Beta-mannanase from Aspergillus terreus and its applicability in depolymerization of mannans and saccharification of lignocellulosic biomass. Biotech 2016, 6, 136. DOI: 10.1007/s13205-016-0454-2 C) Cheng, L.; Duan, S.; Feng, X.; Zheng, K.; Yang, Q.; Liu, Z. Purification and Characterization of a Thermostable Beta-Mannanase from Bacillus subtilis BE-91: Potential Application in Inflammatory Diseases. BioMed Research International 2016, 2016, 1-7. DOI: 10.1155/2016/6380147