We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox: 5VKQ
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
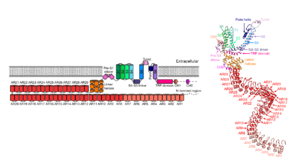
| - | By resolving its structure, researchers highlighted the presence of [https://en.wikipedia.org/wiki/Ankyrin_repeat | + | By resolving its structure, researchers highlighted the presence of 29 [https://en.wikipedia.org/wiki/Ankyrin_repeat ankyrin repeats] per monomer, at the N-terminus. <scene name='10/1083740/Monomer_1_singlear/1'>(Click here to highlight a single ankyrin repeat.)</scene> This is the largest number among TRP channels. An ankyrin repeat is a [https://en.wikipedia.org/wiki/Structural_motif motif] of about 33 amino acids repeated in tandem (i.e., one behind the other). Each repeat adopts a helix-turn-helix conformation. Ankyrin repeats are known to mediate [https://en.wikipedia.org/wiki/Protein%E2%80%93protein_interaction protein-protein interactions] <ref>InterPro (IPR002110). Ankyrin repeat. Available at: https://www.ebi.ac.uk/interpro/entry/InterPro/IPR002110/. Accessed on 2025 June 19.</ref><ref>PMID:17176038</ref>. <scene name='10/1083740/Monomer_1_ardomain/1'>(Click here to highlight the ankyrin repeat domain.)</scene> |
As other TRP channels, NOMPC has a <scene name='10/1083740/Monomer_1_ar_tm/1'>transmembrane domain</scene> characterized by a series of six alpha-helices (S1–S6). Additional <scene name='10/1083740/Monomer_1_ar_tm_lh/1'>linker helices </scene> connect the ankyrin repeat domain and the transmembrane domain. Following the C-terminus portion, there is an intracellular <scene name='10/1083740/Monomer_1_ar_tm_lh_trp/1'>TRP domain</scene>. The rest of the C-terminus is mostly unstructured. | As other TRP channels, NOMPC has a <scene name='10/1083740/Monomer_1_ar_tm/1'>transmembrane domain</scene> characterized by a series of six alpha-helices (S1–S6). Additional <scene name='10/1083740/Monomer_1_ar_tm_lh/1'>linker helices </scene> connect the ankyrin repeat domain and the transmembrane domain. Following the C-terminus portion, there is an intracellular <scene name='10/1083740/Monomer_1_ar_tm_lh_trp/1'>TRP domain</scene>. The rest of the C-terminus is mostly unstructured. | ||
| Line 26: | Line 26: | ||
<scene name='10/1083740/Homotetramer_1/1'>(Click to reset to the tetramer colored view.)</scene> | <scene name='10/1083740/Homotetramer_1/1'>(Click to reset to the tetramer colored view.)</scene> | ||
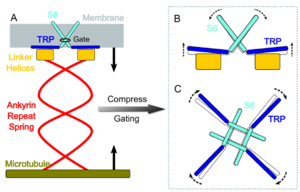
| - | NOMPC gating is thought to be triggered by tethering of its [https://en.wikipedia.org/wiki/Protein_domain | + | NOMPC gating is thought to be triggered by tethering of its ankyrin repeat [https://en.wikipedia.org/wiki/Protein_domain domain] to [https://en.wikipedia.org/wiki/Microtubule microtubules] of the [https://en.wikipedia.org/wiki/Cytoskeleton cytoskeleton]. Structural modelling of this protein suggests that the ankyrin repeat domain in each monomer forms a helical spring. This structure suggests an interaction between the NOMPC and the cytoskeleton, and could suggest the opening of the ion channel in response to cytoskeleton displacement. |
A graphical representation of this proposed mechanism of action is shown in Figure 1. | A graphical representation of this proposed mechanism of action is shown in Figure 1. | ||
| Line 34: | Line 34: | ||
== Biological functions == | == Biological functions == | ||
| - | + | NOMPC is a transient receptor potential mechano-electrical transduction (MET) channel from the fruit fly, ''Drosophila melanogaster''. It is implicated in touch sensation, proprioception and hearing. | |
| - | When this gating mechanism was uncovered | + | The sensation of touch, gravity and sound allows animals to perceive its environment and to navigate through it (literally, since these sensations are required for coordinated body movement). It all starts with the transduction of mechanical stimuli into electrical signals <ref name="hehlert2025" />. Mechanosensory cells mediate this mechano-electrical transduction (MET) through MET channels — ion channels gated directly by mechanical stimuli. When this gating mechanism was uncovered in the 1980’s (in hair cells of the inner ear), it was observed that it requires an elastic element that couples cell deformation to pore formation. |
| - | + | ||
| - | + | ||
=== Locomotion and proprioception === | === Locomotion and proprioception === | ||
| - | NOMPC is essential for proper locomotion and larval crawling | + | NOMPC is essential for proper locomotion and larval crawling <ref name="cheng2010">PMID:20696376</ref>. It mediates [https://en.wikipedia.org/wiki/Proprioception proprioception], which is the sensory input and feedback by which animals control body parts for balance and movement. Its proper function influences the pace of larval crawling. [https://en.wikipedia.org/wiki/Mutation Mutations] in NOMPC can result in severely uncoordinated adult flies and dramatically reduced crawling speed in larvae. NOMPC is required for activating specific sensory neurons during [https://en.wikipedia.org/wiki/Smooth_muscle peristaltic muscle] contractions in larvae, which are critical for locomotion. Its function in sensory neurons is also essential for adult locomotion <ref name="cheng2010" />. |
=== Touch sensation === | === Touch sensation === | ||
| - | NOMPC functions as a mechanotransduction channel for gentle-touch sensation <ref name="yan2013">PMID:23222543</ref>. Loss-of-function mutations in NOMPC abolish mechanoreceptor potentials in fly bristles, and a [https://en.wikipedia.org/wiki/Missense_mutation missense] mutation in the channel alters the adaptation of these potentials. Ectopic expression (i.e. gene expression in a cell, tissue, or developmental stage in which the gene is not usually expressed) of NOMPC can even confer touch sensitivity to normally touch-insensitive neurons <ref name="yan2013" />. | + | NOMPC functions as a mechanotransduction channel for gentle-touch sensation <ref name="yan2013">PMID:23222543</ref>. Loss-of-function mutations in NOMPC abolish mechanoreceptor potentials in [https://en.wikipedia.org/wiki/Bristle_sensilla fly bristles], and a [https://en.wikipedia.org/wiki/Missense_mutation missense] mutation in the channel alters the adaptation of these potentials. Ectopic expression (i.e. gene expression in a cell, tissue, or developmental stage in which the gene is not usually expressed) of NOMPC can even confer touch sensitivity to normally touch-insensitive neurons <ref name="yan2013" />. |
=== Hearing === | === Hearing === | ||
| - | NOMPC is essential for hearing in Drosophila <ref name="jin2016" /><ref name="cheng2010" /> and is particularly important for the function of sound receptors in the fly's antennal hearing organ <ref name="hehlert2025" /><ref name="effertz2011">PMID:21458266</ref>. It is required for the nonlinear mechanical amplification of antennal vibrations, a process that enhances the mechanical sensitivity to faint sounds. Loss of NOMPC function reduces auditory sensitivity and the amplitude of sound-evoked nerve responses <ref name="effertz2011" />. | + | NOMPC is essential for hearing in ''Drosophila'' <ref name="jin2016" /><ref name="cheng2010" /> and is particularly important for the function of sound receptors in the fly's antennal hearing organ <ref name="hehlert2025" /><ref name="effertz2011">PMID:21458266</ref>. It is required for the nonlinear mechanical amplification of antennal vibrations, a process that enhances the mechanical sensitivity to faint sounds. Loss of NOMPC function reduces auditory sensitivity and the amplitude of sound-evoked nerve responses <ref name="effertz2011" />. |
== NOMPC homologs == | == NOMPC homologs == | ||
| Line 60: | Line 58: | ||
* ''Caenorhabditis elegans'' (Nematode worm): [https://www.ncbi.nlm.nih.gov/gene/190574 trp-4] | * ''Caenorhabditis elegans'' (Nematode worm): [https://www.ncbi.nlm.nih.gov/gene/190574 trp-4] | ||
* ''Xenopus laevis'' (African clawed frog): [https://www.ncbi.nlm.nih.gov/gene/734212 nompc] | * ''Xenopus laevis'' (African clawed frog): [https://www.ncbi.nlm.nih.gov/gene/734212 nompc] | ||
| - | * '' | + | * ''Danio rerio'' (Zebrafish): [https://www.ncbi.nlm.nih.gov/gene/368273 trpn1] |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:09, 22 June 2025
| |||||||||||
References
- ↑ Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000 Mar 24;287(5461):2229-34. PMID:10744543 doi:10.1126/science.287.5461.2229
- ↑ 2.0 2.1 Jin P, Bulkley D, Guo Y, Zhang W, Guo Z, Huynh W, Wu S, Meltzer S, Cheng T, Jan LY, Jan YN, Cheng Y. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature. 2017 Jul 6;547(7661):118-122. doi: 10.1038/nature22981. Epub 2017 Jun 26. PMID:28658211 doi:http://dx.doi.org/10.1038/nature22981
- ↑ Mascarenhas NM, Gosavi S. Understanding protein domain-swapping using structure-based models of protein folding. Prog Biophys Mol Biol. 2017 Sep;128:113-120. PMID:27867057 doi:10.1016/j.pbiomolbio.2016.09.013
- ↑ InterPro (IPR002110). Ankyrin repeat. Available at: https://www.ebi.ac.uk/interpro/entry/InterPro/IPR002110/. Accessed on 2025 June 19.
- ↑ Li J, Mahajan A, Tsai MD. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006 Dec 26;45(51):15168-78. PMID:17176038 doi:10.1021/bi062188q
- ↑ Wang Y, Guo Y, Li G, Liu C, Wang L, Zhang A, Yan Z, Song C. The push-to-open mechanism of the tethered mechanosensitive ion channel NompC. Elife. 2021 Jun 8;10:e58388. PMID:34101577 doi:10.7554/eLife.58388
- ↑ 7.0 7.1 7.2 Hehlert P, Effertz T, Gu RX, Nadrowski B, Geurten BRH, Beutner D, de Groot BL, Göpfert MC. NOMPC ion channel hinge forms a gating spring that initiates mechanosensation. Nat Neurosci. 2025 Feb;28(2):259-267. PMID:39762662 doi:10.1038/s41593-024-01849-3
- ↑ 8.0 8.1 8.2 Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010 Aug 12;67(3):373-80. PMID:20696376 doi:10.1016/j.neuron.2010.07.004
- ↑ 9.0 9.1 Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013 Jan 10;493(7431):221-5. PMID:23222543 doi:10.1038/nature11685
- ↑ 10.0 10.1 Effertz T, Wiek R, Göpfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011 Apr 12;21(7):592-7. PMID:21458266 doi:10.1016/j.cub.2011.02.048
- ↑ Schüler A, Schmitz G, Reft A, Özbek S, Thurm U, Bornberg-Bauer E. The Rise and Fall of TRP-N, an Ancient Family of Mechanogated Ion Channels, in Metazoa. Genome Biol Evol. 2015 Jun 22;7(6):1713-27. PMID:26100409 doi:10.1093/gbe/evv091