This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Morphs
From Proteopedia
(→Why Morph? - polishing) |
(→Why Morph? - polishing) |
||
| Line 7: | Line 7: | ||
<table align='right' border='1' width='285'><tr><td> | <table align='right' border='1' width='285'><tr><td> | ||
[[Image:Mage_hb.gif]]</td></tr><tr><td>Toggling between the carbonmonoxy and deoxy conformations of heme in hemoglobin. Convergent stereo snapshot from a Kinemage. (Reload this page to restart the toggling.)</td></tr></table> | [[Image:Mage_hb.gif]]</td></tr><tr><td>Toggling between the carbonmonoxy and deoxy conformations of heme in hemoglobin. Convergent stereo snapshot from a Kinemage. (Reload this page to restart the toggling.)</td></tr></table> | ||
| - | When the differences are small, simply toggling an image between the two states is adequate. [http://kinemage.biochem.duke.edu/ David Richardson's MAGE] (1992 - [http://history.molviz.org history]) supports visual toggling between macromolecular conformations, and hundreds of kinemages take advantage of this capability. At the right is an example from hemoglobin. However, when some of the conformational differences are large, toggling is not sufficient to allow one to follow the transition made by each portion as the image jumps between the two conformations. The purpose of morphing is to smooth the visual transition, making it easier to see the structural | + | When the differences are small, simply toggling an image between the two states is adequate. [http://kinemage.biochem.duke.edu/ David Richardson's MAGE] (1992 - [http://history.molviz.org history]) supports visual toggling between macromolecular conformations, and hundreds of kinemages take advantage of this capability. At the right is an example from hemoglobin. However, when some of the conformational differences are large, toggling is not sufficient to allow one to follow the transition made by each portion as the image jumps between the two conformations. '''The purpose of morphing is to smooth the visual transition, making it easier to see and understand the structural differences between the two empirical conformations.''' |
==Examples of Morphs== | ==Examples of Morphs== | ||
Revision as of 19:49, 13 June 2008
This page is in a rough and incomplete state. I plan to get it into acceptable shape during the next week or two. Emartz 20:48, 23 March 2008 (IST)
Contents |
Why Morph?
Some proteins perform their functions without major conformational changes. In contrast, some proteins must undergo major changes in secondary, tertiary, or quaternary structure in order to perform their functions. In quite a few cases, investigators have succeeded in obtaining empirically determined structures for a protein in two or more conformations. The challenge for visualization is then to be able to follow the changes in each region between the two conformations.
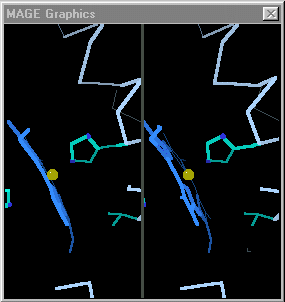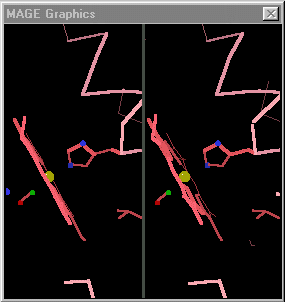 |
| Toggling between the carbonmonoxy and deoxy conformations of heme in hemoglobin. Convergent stereo snapshot from a Kinemage. (Reload this page to restart the toggling.) |
When the differences are small, simply toggling an image between the two states is adequate. David Richardson's MAGE (1992 - history) supports visual toggling between macromolecular conformations, and hundreds of kinemages take advantage of this capability. At the right is an example from hemoglobin. However, when some of the conformational differences are large, toggling is not sufficient to allow one to follow the transition made by each portion as the image jumps between the two conformations. The purpose of morphing is to smooth the visual transition, making it easier to see and understand the structural differences between the two empirical conformations.
Examples of Morphs
- Proton Channels includes a linear interpolation morph of a transmembrane channel opening and closing.
Visualizing Morphs
(to be added)
Morphing Methods
Linear Interpolation
In this method, a series of intermediate models are created in which each atom moves in a straight line from is initial position to its final position. This type of morphing is relatively easy to perform, and often suffices, but also has a number of limitations. When animated, the interpolated intermediate conformations often greatly help in visualizing the differences between the initial and final empirical models. However, otherwise they are otherwise meaningless. Bond lengths and angles become unrealistic, domains may artifactually shrink, expand, or distort, and chains may even pass through each other during the interpolated movements. When there are substantial changes in secondary structure or large movements of domains, linear interpolation becomes unsatisfactory.
A toggler is implemented here for Recoverin. Despite such features as the ability to highlight any arbitrary range of residues, it remains too difficult to follow the changes in secondary and tertiary structure during the large jumps in position. Consequently, a series of intermediate conformations were generated by linear interpolation of alpha carbon positions. This backbone trace "morph" makes it much easier to follow the relations between the two empirical conformations. However, Linear interpolation morphing was first employed by Vonrhein, Schlauderer & Schultz (1995) to create movies of the results of substrate binding to nucleoside monophosphate kinases.
Chemically Possible Intermediates
Plausible intermediate conformations have been calculated by Mark Gerstein and Werner Krebs at Yale University, using a combination of linear interpolation and molecular dynamics. Gerstein has generously made his interpolation data available, and in some cases they are here displayed in this Chime-based Protein Morpher interface. At the Gerstein Lab Homepage you will find movies of plausible morphs in animated GIF's, Quicktime, and VRML. Another source of "chemically reasonable" morphs is the Biomolecular Morphing site by Gerard J. Kleywegt at Uppsala University, Sweden.
Proteopedia Page Contributors and Editors (what is this?)
Eric Martz, Wayne Decatur, Karsten Theis, Joel L. Sussman, Angel Herraez, David Canner, Eran Hodis
